Abstract
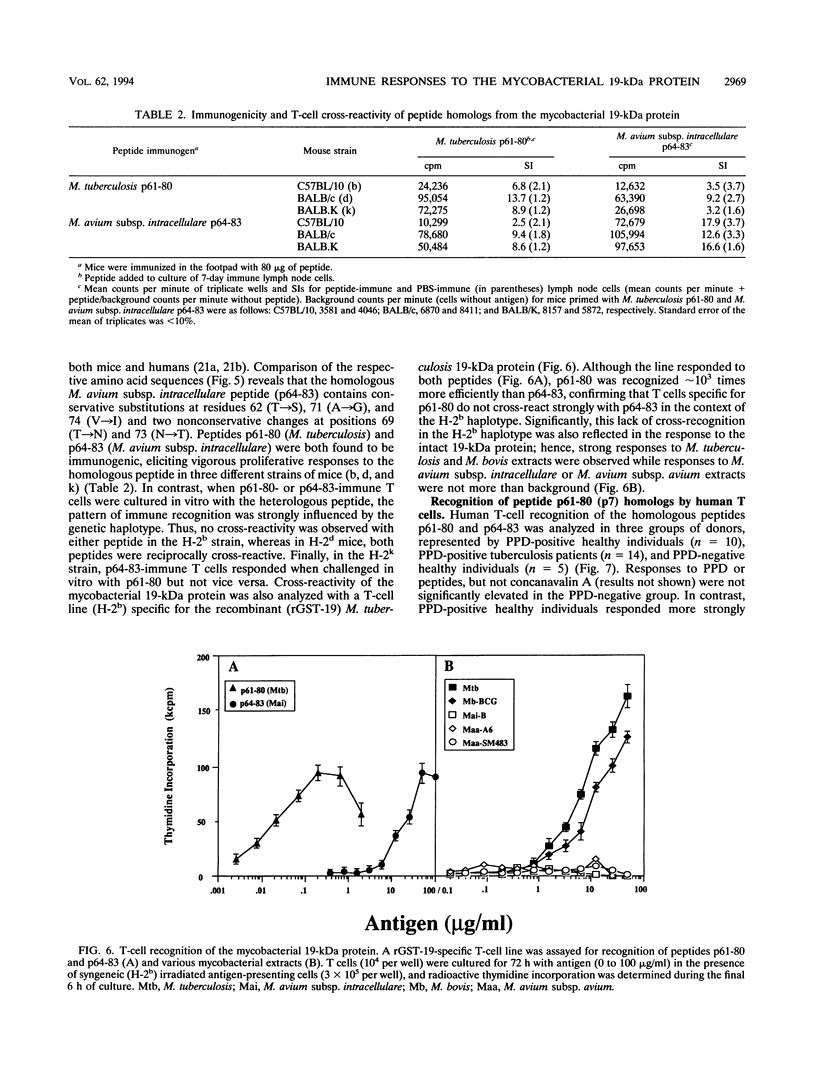
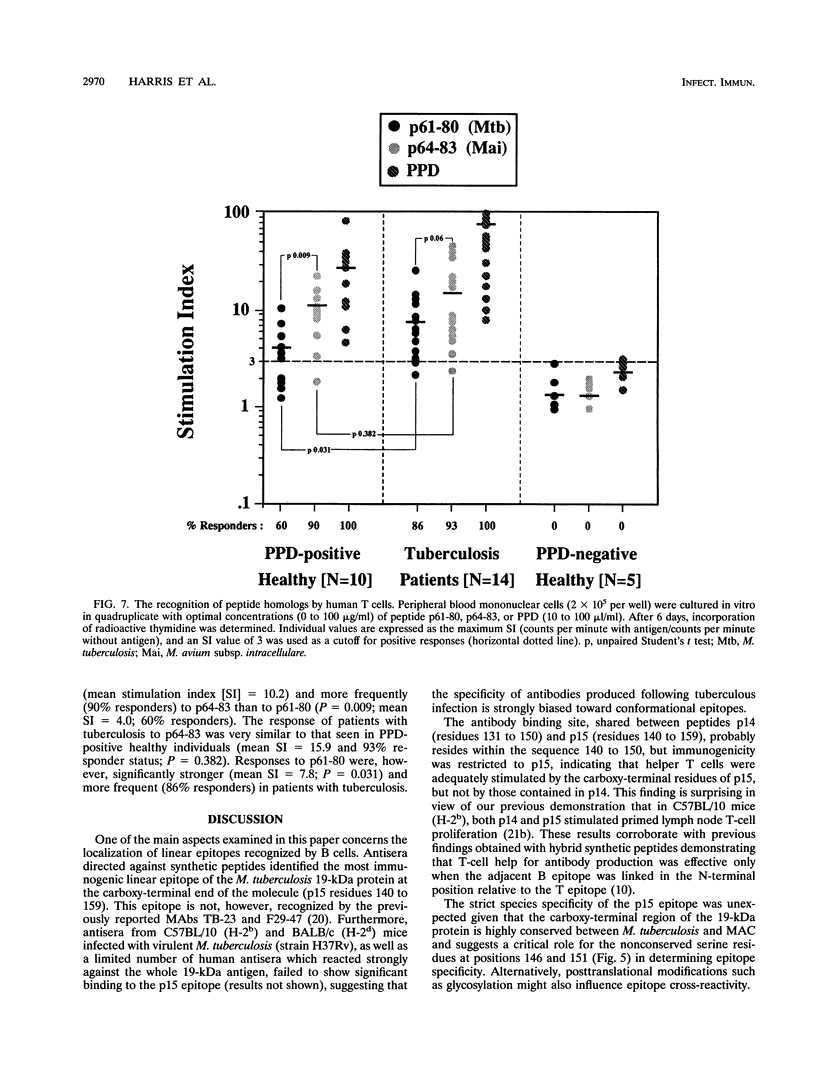
The topography and specificity of B- and T-cell stimulatory epitopes from the 19-kDa protein of Mycobacterium tuberculosis were investigated by using overlapping synthetic peptides. Murine antisera identified two cryptic epitopes (residues 11 to 30 and 61 to 80) and one species-specific immunodominant epitope (residues 140 to 159). Immunoglobulins G1 and G2a antibody isotypes varied for the respective peptide immunogens but without relationship to the T-cell cytokine profiles which were characterized by high gamma interferon and low interleukin 5 levels. Antisera to recombinant M. tuberculosis 19-kDa protein (rGST-19) cross-reacted with homologous proteins of similar size from organisms of the Mycobacterium avium-intracellulare complex. Two-dimensional gel electrophoresis revealed differences in the number, relative mobility, and charge of isoforms of the 19-kDa protein, possibly reflecting posttranslational modifications. The immunodominant T-cell epitope from the M. tuberculosis 19-kDa protein (residues 61 to 80) and the corresponding peptide sequence from Mycobacterium avium subsp. intracellulare (residues 64 to 83), differing at five residues, were both recognized in a genetically permissive manner. Peptides 61-80 and 64-83 stimulated cross-reactive responses in BALB/c (H-2d) mice, while in the C57BL/10 (H-2b) strain, responses to peptide 61-80 were species specific. In purified protein derivative-positive healthy individuals, the M. avium subsp. intracellulare peptide stimulated stronger responses than did the M. tuberculosis peptide, whereas patients with active tuberculosis had enhanced in vitro T-cell responses to both peptides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Andersen P., Ljungqvist L. Structure and function of a 40,000-molecular-weight protein antigen of Mycobacterium tuberculosis. Infect Immun. 1992 Jun;60(6):2317–2323. doi: 10.1128/iai.60.6.2317-2323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbridge K. R., Booth R. J., Watson J. D., Lathigra R. B. Nucleotide sequence of the 19 kDa antigen gene from Mycobacterium tuberculosis. Nucleic Acids Res. 1989 Feb 11;17(3):1249–1249. doi: 10.1093/nar/17.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbridge K. R., Bäckström B. T., Liu H. X., Vikerfors T., Englebretsen D. R., Harding D. R., Watson J. D. Mapping of T helper cell epitopes by using peptides spanning the 19-kDa protein of Mycobacterium tuberculosis. Evidence for unique and shared epitopes in the stimulation of antibody and delayed-type hypersensitivity responses. J Immunol. 1992 Apr 1;148(7):2248–2255. [PubMed] [Google Scholar]
- Ashbridge K. R., Prestidge R. L., Booth R. J., Watson J. D. The mapping of an antibody-binding region on the Mycobacterium tuberculosis 19 kilodalton antigen. J Immunol. 1990 Apr 15;144(8):3137–3142. [PubMed] [Google Scholar]
- Bothamley G., Batra H., Ramesh V., Chandramui A., Ivanyi J. Serodiagnostic value of the 19 kilodalton antigen of Mycobacterium tuberculosis in Indian patients. Eur J Clin Microbiol Infect Dis. 1992 Oct;11(10):912–915. doi: 10.1007/BF01962372. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Ivanyi J. Genetic influences on the immune repertoire following tuberculous infection in mice. Immunology. 1990 Sep;71(1):113–119. [PMC free article] [PubMed] [Google Scholar]
- Caulada-Benedetti Z., al-Zamel F., Sher A., James S. Comparison of Th1- and Th2-associated immune reactivities stimulated by single versus multiple vaccination of mice with irradiated Schistosoma mansoni cercariae. J Immunol. 1991 Mar 1;146(5):1655–1660. [PubMed] [Google Scholar]
- Collins M. E., Patki A., Wall S., Nolan A., Goodger J., Woodward M. J., Dale J. W. Cloning and characterization of the gene for the '19 kDa' antigen of Mycobacterium bovis. J Gen Microbiol. 1990 Jul;136(7):1429–1436. doi: 10.1099/00221287-136-7-1429. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Ivanyi J., Young D. B., Lamb J. R., Syred A. D., Francis M. J. Orientation of epitopes influences the immunogenicity of synthetic peptide dimers. Eur J Immunol. 1988 Dec;18(12):2015–2019. doi: 10.1002/eji.1830181222. [DOI] [PubMed] [Google Scholar]
- DeKruyff R. H., Mosmann R. R., Umetsu D. T. Induction of antibody synthesis by CD4+ T cells: IL 5 is essential for induction of antigen-specific antibody responses by TH2 but not TH1 clones. Eur J Immunol. 1990 Oct;20(10):2219–2227. doi: 10.1002/eji.1830201010. [DOI] [PubMed] [Google Scholar]
- Elsaghier A., Nolan A., Allen B., Ivanyi J. Distinctive western blot antibody patterns induced by infection of mice with individual strains of the Mycobacterium avium complex. Immunology. 1992 Jul;76(3):355–361. [PMC free article] [PubMed] [Google Scholar]
- Faith A., Moreno C., Lathigra R., Roman E., Fernandez M., Brett S., Mitchell D. M., Ivanyi J., Rees A. D. Analysis of human T-cell epitopes in the 19,000 MW antigen of Mycobacterium tuberculosis: influence of HLA-DR. Immunology. 1991 Sep;74(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Fifis T., Costopoulos C., Radford A. J., Bacic A., Wood P. R. Purification and characterization of major antigens from a Mycobacterium bovis culture filtrate. Infect Immun. 1991 Mar;59(3):800–807. doi: 10.1128/iai.59.3.800-807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Fox B. S. Antibody responses to a cytochrome c peptide do not correlate with lymphokine production patterns from helper T-cell subsets. Immunology. 1992 Jan;75(1):164–169. [PMC free article] [PubMed] [Google Scholar]
- Garbe T., Harris D., Vordermeier M., Lathigra R., Ivanyi J., Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993 Jan;61(1):260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsia R. J., Hellqvist L., Booth R. J., Radford A. J., Britton W. J., Astbury L., Trent R. J., Basten A. Homology of the 70-kilodalton antigens from Mycobacterium leprae and Mycobacterium bovis with the Mycobacterium tuberculosis 71-kilodalton antigen and with the conserved heat shock protein 70 of eucaryotes. Infect Immun. 1989 Jan;57(1):204–212. doi: 10.1128/iai.57.1.204-212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. P., Vordermeier H. M., Friscia G., Román E., Surcel H. M., Pasvol G., Moreno C., Ivanyi J. Genetically permissive recognition of adjacent epitopes from the 19-kDa antigen of Mycobacterium tuberculosis by human and murine T cells. J Immunol. 1993 Jun 1;150(11):5041–5050. [PubMed] [Google Scholar]
- Harris D. P., Vordermeier H. M., Roman E., Lathigra R., Brett S. J., Moreno C., Ivanyi J. Murine T cell-stimulatory peptides from the 19-kDa antigen of Mycobacterium tuberculosis. Epitope-restricted homology with the 28-kDa protein of Mycobacterium leprae. J Immunol. 1991 Oct 15;147(8):2706–2712. [PubMed] [Google Scholar]
- Ivanyi J., Sharp K. Control by H-2 genes of murine antibody responses to protein antigens of Mycobacterium tuberculosis. Immunology. 1986 Nov;59(3):329–332. [PMC free article] [PubMed] [Google Scholar]
- Jackett P. S., Bothamley G. H., Batra H. V., Mistry A., Young D. B., Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Rees A. D., Bal V., Ikeda H., Wilkinson D., De Vries R. R., Rothbard J. B. Prediction and identification of an HLA-DR-restricted T cell determinant in the 19-kDa protein of Mycobacterium tuberculosis. Eur J Immunol. 1988 Jun;18(6):973–976. doi: 10.1002/eji.1830180623. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Bai G. H., Rouse D. A., Armoa G. R., Chuidian F., Nair J., Morris S. L. A comparison of the T cell delayed-type hypersensitivity epitopes of the 19-kD antigens from Mycobacterium tuberculosis and Myco. intracellulare using overlapping synthetic peptides. Clin Exp Immunol. 1993 Aug;93(2):172–177. doi: 10.1111/j.1365-2249.1993.tb07961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Fong T. A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989 Jan 17;116(2):151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- Nair J., Rouse D. A., Morris S. L. Nucleotide sequence analysis and serologic characterization of the Mycobacterium intracellulare homologue of the Mycobacterium tuberculosis 19 kDa antigen. Mol Microbiol. 1992 Jun;6(11):1431–1439. doi: 10.1111/j.1365-2958.1992.tb00863.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer C., Murray J., Madri J., Bottomly K. Selective activation of Th1- and Th2-like cells in vivo--response to human collagen IV. Immunol Rev. 1991 Oct;123:65–84. doi: 10.1111/j.1600-065x.1991.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Rees A. D., Faith A., Roman E., Ivanyi J., Wiesmuller K. H., Moreno C. The effect of lipoylation on CD4 T-cell recognition of the 19,000 MW Mycobacterium tuberculosis antigen. Immunology. 1993 Nov;80(3):407–414. [PMC free article] [PubMed] [Google Scholar]
- Schumacher J. H., O'Garra A., Shrader B., van Kimmenade A., Bond M. W., Mosmann T. R., Coffman R. L. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988 Sep 1;141(5):1576–1581. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Surcel H. M., Troye-Blomberg M., Paulie S., Andersson G., Moreno C., Pasvol G., Ivanyi J. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994 Feb;81(2):171–176. [PMC free article] [PubMed] [Google Scholar]
- Tan P. L., Farmiloe S., Young J., Watson J. D., Skinner M. A. Lymphocyte responses to DR4/1-restricted peptides in rheumatoid arthritis. The immunodominant T cell epitope on the 19-kd Mycobacterium tuberculosis protein. Arthritis Rheum. 1992 Dec;35(12):1419–1426. doi: 10.1002/art.1780351204. [DOI] [PubMed] [Google Scholar]
- Verbon A., Kuijper S., Jansen H. M., Speelman P., Kolk A. H. Antibodies against secreted and non-secreted antigens in mice after infection with live Mycobacterium tuberculosis. Scand J Immunol. 1992 Sep;36(3):371–384. doi: 10.1111/j.1365-3083.1992.tb02951.x. [DOI] [PubMed] [Google Scholar]
- Young D. B., Garbe T. R. Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect Immun. 1991 Sep;59(9):3086–3093. doi: 10.1128/iai.59.9.3086-3093.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Garbe T. R. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991 Jan;142(1):55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]