Abstract
Kingella kingae is an emerging paediatric pathogen that most commonly is associated with relatively benign osteoarticular disease in children. This report concerns a 1-year-old child with Kingella kingae endocarditis and perivalvular abscess complicated by septic cerebral emboli and osteomyelitis leading to long-term neurological sequelae, highlighting the capacity of this organism to cause severe invasive disease in children.
Background
Kingella kingae is increasingly being recognised as cause of disease in childhood. It commonly presents as osteoarticular infections, which are often benign. To our knowledge we present the first case of K kingae endocarditis with perivalvular abscess in a previously well child. The aggressive nature of this infection is described, and alerts the reader to this emerging pathogen, some unique microbiological features and its ability to cause severe invasive disease.
Case presentation
In December 2008, a 14-month-old Caucasian boy presented to our institution with a 24-h history of fever, diarrhoea and vomiting. In the week prior to presentation, he and his two siblings had been unwell with fever and cough. There was no history of recent travel and his immunisations were up to date according to Australian National guidelines (http://immunise.health.gov.au/internet/immunise/publishing.nsf/Content/nips2).
On examination he was lethargic and looked unwell. His temperature was 38.2°C, heart rate 190 per min, respiratory rate 46 per min, blood pressure 99/44 mm/Hg and oxygen saturation 91% in room air. He had cool peripheries with a capillary refill time of 4 s. There was no visible rash. Auscultation revealed a grade 3/6 ejection systolic murmur, heard throughout the praecordium but loudest in the second intercostal space right parasternal area. The liver edge was palpable 3 cm below the costal margin. There was no splenomegaly or lymphadenopathy. No peripheral stigmata of endocarditis were identified.
Initial investigations showed a haemoglobin level of 8.9 g/dl, white blood cell count 16.2×109/litre (absolute neutrophil count 10.2×109/litre) and platelet count 311×109/litre. C reactive protein was 78 mg/litre (normal <8 mg/litre). Liver transaminases, urea and creatinine were normal. Coagulation studies revealed an international normalised ratio (INR) of 1.3 but were otherwise unremarkable. A blood culture was taken and he was given intravenous cefotaxime, gentamicin and vancomycin for presumed sepsis. Two fluid boluses were administered but persistent haemodynamic instability and deterioration in his level of consciousness, necessitated a transfer to the intensive care unit. He was subsequently intubated and given inotropes.
Investigations
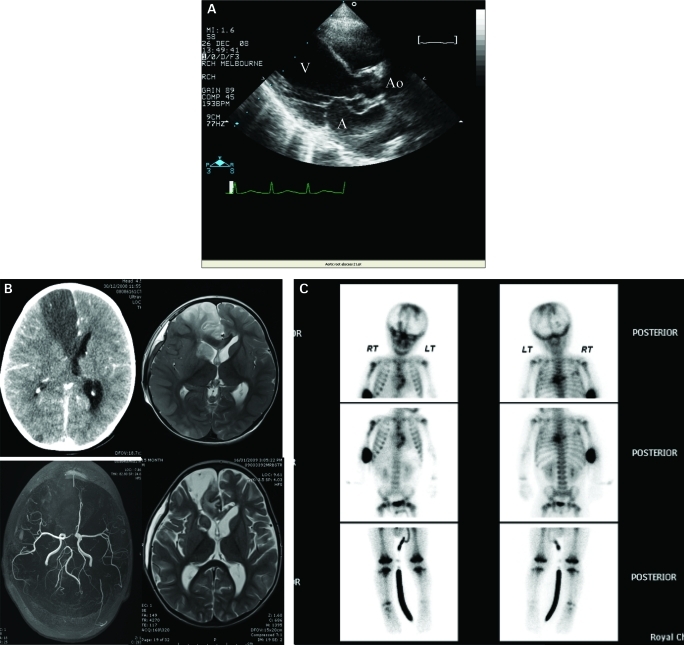
A chest radiograph showed increased interstitial markings consistent with acute pulmonary oedema and right middle lobe collapse. With these findings and an inotropic requirement a transthoracic echocardiogram was arranged. This revealed an aortic valve vegetation, severe aortic regurgitation and destruction of the non-coronary cusp. In addition, a lesion consistent with an aortic perivalvular abscess was visualised creating a fistula into the left atrium (fig 1A). He underwent urgent aortic root replacement with an aortic homograft and repair of the anterior mitral valve leaflet with an autologous pericardial patch. Culture of the aortic valve tissue was negative.
Figure 1.
A. longitudinal axis view showing perivalvular abscess protruding into left atrium. A, left atrium; Ao, aorta; V, left ventricle. B. Top left, CT scan. Top right/bottom left, MRI and magnetic resonance angiography. Bottom right, follow-up MRI at day 22 showing residual defect in right frontal lobe and caudate with resolved oedema. C. Bone scan.
A paediatric blood culture bottle (BacT-Alert, bioMériex, Durham, North Carolina, USA) became positive after 24 h incubation at 35°C. Gram-negative coccobacilli were seen on the wet preparation and Gram stain. After 48 h, growth on the chocolate and horse blood agar revealed the causative organism.
K kingae was identified on subculture and confirmed using the Remel RapID NH (Remel, Lenexa, Kansas, USA). Sensitivity testing by disc diffusion on Mueller–Hinton agar indicated susceptibility to ampicillin with an MIC of 0.064 μl/ml (e test). The antibiotic regimen was rationalised to benzylpenicillin and gentamicin.
Following an initial period of stability, he developed seizures on the fourth postoperative day. Cranial CT and subsequent MRI showed oedema with midline shift and suggested right anterior cerebral artery territory infarction with involvement of the right caudate nucleus. MRI angiography revealed complete obstruction of the right anterior cerebral artery (fig 1B).
Phenytoin was given and in view of the radiological evidence of intracranial hypertension a decompressive craniectomy performed. Additional investigations to identify other potential foci were performed and included a bone scan. This showed increased radiopharmaceutical accumulation in a multifocal distribution suggesting osteomyelitis in the metaphyses of both humeri, distal femurs and proximal tibiae, ribs and spine (fig 1C). Fundoscopy was unremarkable.
Outcome and follow-up
Cardiovascular stability permitted weaning of sedation and respiratory support that subsequently enabled extubation on day 10. Upon weaning sedation, an evolving left-sided hemiparesis became apparent. Clinical progress included 8 weeks of inpatient rehabilitation and management of residual cardiac failure. He completed a 6-week course of benzylpenicillin and a reconstructive cranioplasty was performed 12 weeks post craniectomy. A detailed immunological evaluation revealed no underlying immune deficiency. On follow-up at 4 months, he had a residual left-sided hemiparesis with associated cognitive dysfunction.
Discussion
Once a rarely noted pathogen, K kingae, a member of the HACEK (for Haemophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella) group of organisms, is now increasingly recognised as a cause of osteomyelitis, septic arthritis and endocarditis in children.1–3 However, to our knowledge, this is the first reported case of endocardial abscess associated with K kingae infection in a child. The case highlights the aggressive nature of left-sided K kingae endocarditis, the propensity for embolic phenomena and the potential for long-term complications.
First described by Elizabeth King in 1960, K kingae is a Gram-negative coccobacillus that is known to colonise the nasopharynx with a preferred anatomical niche in the tonsillar bed.3 Typically, infants become colonised from 6 months of age and person-to-person transmission has been documented. Invasive disease most commonly occurs in children less than 5 years of age.2 Musculoskeletal infections, particularly septic arthritis and osteomyelitis, represent the most common clinical manifestations of disease and, unlike endocarditis, are considered to be relatively benign.
Typically fastidious, K kingae requires blood agar for growth on solid media. It is β-haemolytic and characteristically shows pitting within the agar. In our case, isolation of K kingae within 48 h was facilitated by the BacT-Alert blood culture system. In disease without bacteraemia, direct inoculation of clinical samples (for example, pus or synovial fluid) into BacT-Alert blood culture bottles has been shown to increase the yield, particularly in osteoarticular manifestations of disease.4
Paediatric endocarditis is predominantly caused by Staphylococcus aureus and viridans group streptococci with HACEK organisms being responsible for around 5%. Prior experience with K kingae endocarditis has been limited primarily to case reports5,6 where infection has typically been accompanied by valvular destruction, cardiac failure with embolic phenomena.2 In a review of HACEK endocarditis, K kingae was identified as the causative agent in over a third of cases, with over 50% of these occurring in the setting of pre-existing cardiac abnormalities.6 Abscess formation was not reported and the overall mortality in HACEK endocarditis was reported to be as high as 14%.6
Endocardial abscess formation and fistulisation, though relatively common in adults,7 is rarely seen in children8–10 where staphylococcal and streptococcal organisms have been almost exclusively implicated. Abscess formation has non-specific features; however, persistent fever, ongoing bacteraemia and new conduction disturbance should alert the doctor to this possibility. Surgery is also indicated for intractable sepsis, cardiac failure or significant embolic phenomenon however the optimal timing for surgical intervention remains controversial.10
K kingae is susceptible to a wide antibiotic spectrum suggesting that treatment failure is unlikely in the empiric treatment of sepsis. However, as our case illustrates, even with appropriate antibiotic treatment, the potential for embolic complications exists.
This emerging paediatric pathogen should be considered in the child with infective endocarditis as should perivalvular abscess in the context of cardiogenic shock, persistent bacteraemia or new conduction disturbance.
Learning points
Kingella kingae, a member of the HACEK (for Haemophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella) group of organisms, is an emerging paediatric pathogen.
K kingae endocarditis with perivalvular abscess has the potential for severe embolic complications.
Endocarditis in the presence of cardiogenic shock should alert the clinician to the possibility of abscess formation.
Direct inoculation of clinical samples into a BacT-Alert blood culture bottle increases the yield of growth of K kingae.
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Yagupsky P. Kingella kingae: from medical rarity to an emerging clinical pathogen. Lancet Infect Dis 2004; 4: 358–67 [DOI] [PubMed] [Google Scholar]
- 2.Yagupsky P, Dagan R. Kingella kingae: an emerging cause of invasive infections in young children. Clin Infect Dis 1997; 24: 860–6 [DOI] [PubMed] [Google Scholar]
- 3.Yagupsky P, Porat N, Pinco E. Pharyngeal colonisation by Kingella kingae with invasive disease. Ped Infect Dis J 2009; 28: 155–7 [DOI] [PubMed] [Google Scholar]
- 4.Yagupsky P, Dagan R, Howard CB, et al. High prevalence of Kingella Kingae in joint fluid from children with septic arthritis revealed by the BACTEC blood culture system. J Clin Microbiol 1992; 30: 1278–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubnov-Raz G, Scheuerman O, Chodick G, et al. Invasive Kingella kingae infections in children: clinical and laboratory characteristics. Pediatrics 2008; 122: 1305–9 [DOI] [PubMed] [Google Scholar]
- 6.Feder HM, Roberts JC, Salazar JC, et al. HACEK endocarditis in infants and children: two cases and a literature review. Pediatr Infect Dis J 2003; 22: 557–62 [DOI] [PubMed] [Google Scholar]
- 7.Blumberg EA, Karalis DA, Chandrasekeran K, et al. Endocarditis associated paravalvular abscesses. Chest 1995; 107: 898–903 [DOI] [PubMed] [Google Scholar]
- 8.Koch A, Cesnjevar R, Buheitel G, et al. Aortic root abscess complicated by fistulization and intracerebral haemorrhagic infarction. Petiatr Cardiol 2003; 24: 576–80 [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi R, deLeval M, Sullivan D. Urgent homograft aortic root replacement for aortic root abscess in infants and children. Heart 1999; 81: 62–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah FS, Fennelly G, Weingarten-Arams J, et al. Endocardial abscess in children: case report and review of the literature. Clin Infect Dis 1999; 29: 1478–82 [DOI] [PubMed] [Google Scholar]