Abstract
A 4-year-old severely disabled boy with a congenital myopathy developed profuse diarrhoea with hypernatraemia (plasma Na 157 mmol/l). The initial blood urea, serum creatinine and urine output were within normal limits. Despite corrective measures within a hospital setting, the patient’s serum sodium peaked at 202 mmol/l. A high fractional excretion of sodium (FE Na) in the context of dehydration and normal renal function was suggestive of a high sodium load. Subsequent investigations revealed an unusual combination of valproate-induced Fanconi syndrome, nephrogenic diabetes insipidus and excess sodium load. The case illustrates why severe hypernatraemia in children is such a diagnostic challenge.
BACKGROUND
Our case highlights the extraordinary difficulties encountered if hypernatraemia occurs from a combination of factors in someone who cannot indicate thirst. It can be extremely dangerous to pursue a path of salt poisoning, from a legal and moral standpoint, when subtle renal tubular abnormalities have not been ruled out. Sodium valproate is a commonly used antiepileptic and our case is a reminder of the value of monitoring renal tubular function, especially in the severely neurologically impaired population who are on this medication.
CASE PRESENTATION
A 4-year-old boy of Asian origin with a congenital myopathy was referred to a tertiary paediatric high dependency unit for respiratory support. His parents were first cousins and he had an older sister with a similar myopathy that despite investigations had eluded a conclusive diagnosis. At the age of 9 months the child had been left with significant hypoxic brain damage following a life threatening episode of pulmonary aspiration. Since then he had required nasogastric feeding and non-invasive ventilation via tracheostomy. He was completely immobile and only winced in response to deep painful stimulation.
During a hospital admission 4 months prior to the current one, he was noted to have glycosuria and a very low serum phosphate (0.6 mmol/l). He was started on phosphate supplements. There was a history of intermittent diarrhoea over the 3 months prior to admission and he had been fed on a volume of 65 ml/kg/day of Pediasure exclusively, with water being used only to flush the nasogastric tube.
On this admission he was diagnosed with a lower respiratory tract infection for which he was started on antibiotics. Subsequently he developed severe diarrhoea and hypernatraemia (plasma sodium (P Na) 157 mmol/l). Nasogastric rehydration was commenced using Dioralyte and Pediasure (table 1). Twenty four hours later his serum sodium continued to rise (162–167 mmol/l). Severe diarrhoeal losses persisted and his feeds were stopped. Despite receiving standard treatment of intravenous 0.9% saline,1 the P Na concentration continued to rise over the next 48 h reaching a maximum of 202 mmol/l. Tests revealed no obvious renal dysfunction (urine output 1.4 ml/kg/h, blood urea 2 mmol/l, serum creatinine 42 μmol/l). The fractional excretion of sodium (FE Na) was extraordinarily high at 6% with a urine osmolality at 430 mosm/l which in the absence of renal dysfunction suggested salt poisoning.
Table 1.
Fluid balance chart
| Input | Fluids used | Urine output | Weight | |
| Preadmission | 65 ml/kg/day | Pediasure (Na content: 300 mg in 500 ml) | No H/O polyuria | 16 kg |
| Day of admission | 65 ml/kg/day | Pediasure | 2.5 ml/kg/h | 15 kg |
| Day 2 | 100 ml/kg/day | 50% Pediasure and 50% Dioralyte | 1.2 ml/kg/h | |
| Day 3 | 120 ml/kg/day | Pediasure/Dioralyte and 0.45% saline | 2 ml/kg/h | |
| Day 4 | 120 ml/kg/day | 0.9% Saline | 1.4 ml/kg/h | |
| Day 5 | 120 ml/kg/day | 0.9% Saline | 1.7 ml/kg/h | |
| Day 6 | 120 ml/kg/day | 5% Dextrose | 0.9 ml/kg/h | |
| Day 7 | 120 ml/kg/day | Pediasure | 0.9 ml/kg/h | |
| Day 15 | 150 ml/kg/day | 40% Pediasure and 60% water | 4.5 ml/kg/h | 17 kg |
His parents denied the use of any salt preparation and we had no reason to doubt them.
However, the child was receiving several sodium containing medications and fluids, with calculations revealing a high sodium intake (table 2): sodium containing medications included sodium valproate, Gaviscon and Phosphate-Sandoz, while fluids used included Dioralyte, Pediasure, Gelofusin, and 0.45% and 0.9% saline
Table 2.
Sodium input
| Na (mmol/kg/day) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
| From medication | 6.2 | 6.2 | 6.2 | 6.2 | 6.2 | 4.8 | None |
| From fluids | 1.7 | 2.3 | 8.3 | 16.6 | 15.5 | 6.9 | 6.2 |
| Total | 7.9 | 8.5 | 14.5 | 22.8 | 21.7 | 11.7 | 6.2 |
Hypernatraemia was eventually corrected by stopping all sodium containing medications and fluids (for a 24 h period the patient received solely 5% glucose). However, the rate of fall in serum sodium was slower than we expected for a case of isolated salt poisoning.
Further scrutiny allowed for evaluation of other renal abnormalities:
Urine osmolality of 430 mosm/kg was inappropriately low in the context of a serum osmolality of 390 mosm/kg. Random urine osmolalities were persistently low (120–350 mosm/kg).
His initial urea and creatinine were 2 mmol/l and 40 μmol/l. Although these absolute figures are not abnormally high, they were higher than his preadmission baseline values of 1 mmol/l and <30 μmol/l, respectively. This can be interpreted as abnormal in the context of extreme immobility and poor muscle bulk.
The figures for the urea and creatinine peaked to a maximum of 4 mmol/l and 60 μmol/l, respectively.
Glycosuria with normal blood sugar persisted throughout.
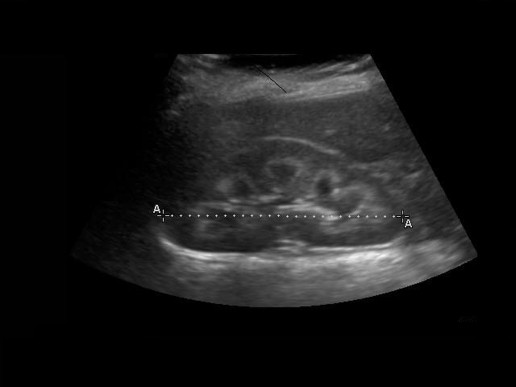
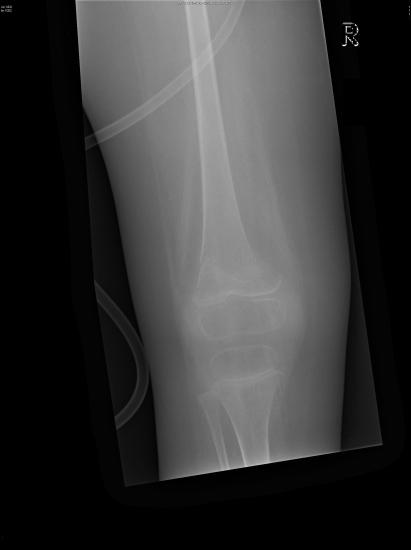
Hyperchloraemic non-anion gap metabolic acidosis was initially attributed to gut losses but persisted long after the diarrhoea subsided. The urine pH of 7.5 (serum pH 7.2) and the positive urinary anion gap2,3 revealed an underlying distal renal tubular acidosis. Detailed urine examination showed aminoaciduria, glycosuria, proteinuria, kaliuria and phosphaturia suggestive of Fanconi syndrome. Renal ultrasound showed bilateral nephrocalcinosis (fig 1). During his hospital stay the patient also sustained three pathological fractures from minimal trauma (fig 2) which we attribute to severe osteopenia from prolonged immobility and hypophosphataemia.
Figure 1.
Renal ultrasound
Figure 2.
Pathological fracture of the right femur
INVESTIGATIONS
Urine (reference range in brackets)
Persistently low osmolality, usually around 200 mosm/kg: there was no response to desmopressin challenge.
pH 7–7.5
Glycosuria
Generalised aminoaciduria
The following are expressed as μmol/mmol keratinise:
Glutamic acid 1785 (0–3)
Serine 11 066 (27–55)
Glycine 20 586 (123–589)
Glutamine 20 586 (37–97)
Taurine 206 (40–176)
Histidine 2944 (51–356)
Citrulline 298 (1.1–8)
Threonine 6989 (12–27)
Alanine 31402 (66–77)
Tyrosine 3140 (5–16)
Valine 3260 (1.1–8)
Methionine 213 (2–10)
Cystine 438 (4–16)
Isoleucine 285 (1.1–6)
Leucine 399 (2–12)
Lysine 2798 (10–53)
Other results were as follows:
Proteinuria 1154 mg/l (0–100)
Urine N-acetyl glucosamine/creatinine ratio: 788 units/mmol (normal reference 2–22 units/mmol; urinary creatinine 1.2 mmol/l)
Urine retinol binding protein/creatinine ratio 102 333 μg/mmol (normal reference 4.5–89 μg/mmol; urinary creatinine 1.2 mmol/l)
Potassium inappropriately high at 63 mmol/l when serum K was 3.1 mmol/l (<20 mmol/l)
Phosphate 6.6 mmol/l, when plasma phosphate was 0.6 mmol/l
Tubular reabsorption of phosphate: could not be estimated as the urine sample was too dilute
24 h urinary calcium: 0.1 mmol/kg/24 h (0–0.15)
Anion gap 13 mmol/l
Blood (reference range in brackets)
Potassium 2.5–3.0 mmol/l (3.5–5.0)
Phosphate 0.6 mmol/l (1–2)
Bicarbonate 15–18 mmol/l (22–25)
pH 7.15–7.2
Calcium: 2.2–2.5 mmol/l (2.12–2.62)
PTH: 1.0 U/l (1.3–7.6)
25–OH vitamin D2: 8.5 ng/ml and 25-OH vitamin D3: 2.4 ng/ml giving a total 25-OH vitamin D of 10.9 ng/ml, indicating deficiency
Reference intervals for total 25-OH vitamin D as below:
<6 ng/ml indicates severe deficiency
6–12 ng/ml indicates deficiency
12–20 ng/ml indicates insufficiency
20–60 ng/ml indicates adequate status
>60 ng/ml indicates toxicity
1,25 Vitamin D: 56 pmol/l (43–144)
Cortisol: 258 nmol/l (180–620)
Alkaline phosphate: 1246 IU/l (250–720)
Albumin 30–39 g/l (35–50)
Magnesium 0.9 mmol/l (0.75–1.05)
Urea and creatinine when well hydrated: 1 mmol/l and <30 μmol/l, respectively
GFR: 75 ml/min/1.73 m2
Osmolality: variable based on hydration 290–390 mosm/l
Anion gap 10–13 mmol/l
Ultrasound of the kidneys
Large kidneys bilaterally, 8.6 cm on the right and 9.1 cm on the left with marked bilateral medullary nephrocalcinosis. There was no hydronephrosis and the bladder was empty (fig 1).
CT brain
Preservation of grey/white matter with no mass lesions seen. There was dilatation of the ventricles which was not unexpected considering the patient’s neurological status.
TREATMENT
Discontinuation of sodium valproate as our patient had been seizure-free for more than 2 years
Optimisation of fluid intake to match the relatively high fixed urine output of 2–2.5 l/day (700 ml of Pediasure and 1750 ml of water)
6 mmol/kg/day potassium supplements were required to maintain a serum potassium of 3.5 –4.0 mmol/l
5 mmol/kg/day bicarbonate supplements were required to maintain a serum bicarbonate of 23–25 mmol/l
3 mmol/kg/day phosphate supplements to normalise serum phosphate to around 1.9 mmol/l
1 alpha calcidol supplements
Low sodium diet (less than 1 mmol/kg/day).
OUTCOME AND FOLLOW-UP
Following treatment our patient’s biochemistry stayed consistently normal. The family were advised to increase free water intake during diarrhoeal illnesses. His weight on discharge was 19 kg.
Blood results 3 months after the original admission
pH 7.33
Bicarbonate 23 mmol/l
Sodium 135 mmol/l
Potassium 3.9 mmol/l
Osmolality 278 mosm/kg
Phosphate 1.9 mmol/l
DISCUSSION
We believe this case merits discussion for two reasons. Firstly, to highlight the difficulties encountered in unravelling the aetiology of hypernatraemia, and secondly to describe an unusual side effect of a commonly used antiepileptic drug, sodium valproate.4,5
Hypernatraemia occurs for one or more of the following reasons:
Salt overload
Hypotonic losses (diarrhoea)
Renal concentrating defects.
This case of hypernatraemia was particularly challenging because while the patient had a clear history of diarrhoea, we were initially unaware that there was an underlying significant renal tubular defect. An extremely high FE Na of 6% (table 3) in the context of hypernatraemia and normal renal function was strongly suggestive of salt poisoning.6,7 This later revealed itself to be due to a combination of sodium valproate-induced Fanconi syndrome, nephrogenic diabetes insipidus and excess sodium content in medication and fluids.
Table 3.
Sodium excretion
| Day 4 | Day 9 | Day 14 | Day 27 | ||||
| fractional excretion of sodium ( mmol/l) | 193 | 163 | 146 | 137 | |||
| FE Na% | 6.2 | 3.8 | 3.9 | 0.8 | |||
| Urine Osm (mosm/kg) | 430 | 350 | 240 | 130 | |||
| Urine Na (mmol/l) | 133 | 24 | 106 | 32 |
| Day 2 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 15 | |
| Blood urea (mmol/l) | 1.4 | 2.0 | 3.7 | 4.1 | 6.0 | 5.1 | 1.1 |
| Serum creatinine (μmol/l) | 44 | 42 | 63 | 60 | 60 | 43 | 31 |
FE Na, fractional excretion of sodium; P Na, plasma sodium.
It has occurred to us that if this child had died early in admission from his extreme hypernatraemia, we may not have been able to make this diagnosis. We suggest that in cases of hypernatraemic dehydration it is vital that paired urine and serum sodium and osmolalities are obtained and FE Na is calculated as early as possible (table 4).
Table 4.
Differentiation of various aetiologies of hypernatraemia
| High P Na | U Osm | FE Na |
| Dehydration, prerenal failure | High (>600) | <1% |
| Dehydration, renal failure | Low (<400) | >3% |
| Salt poisoning | High | >3% |
| Poorly concentrated urine | Low | <1% |
Table reproduced with permission from referring to figs 1 and 3 of the article by Coulthard.6
The unusual combination of sodium valproate-induced Fanconi syndrome, nephrogenic diabetes insipidus and salt overload combined to cause severe and resistant hypernatraemia in this case.
Sodium valproate is a rare but recognised cause of Fanconi syndrome, a proximal renal tubular acidosis.4,5 A PubMed based literature search revealed that 14 such cases have been published worldwide so far. In the case series published by Knorr et al,4 children had been receiving sodium valproate for periods ranging from 10 months to 8 years when Fanconi syndrome was detected. In the same series they note that symptoms were fully reversible within 2–14 months of stopping the drug. Our patient had been on sodium valproate for 3 years.
We attribute the nephrogenic diabetes insipidus, a distal renal concentrating defect, to long standing acidosis and hypokalaemia from Fanconi syndrome.
Prior to admission, this child was receiving 7.9 mmol/kg/day of sodium and this has since been decreased to less than 1 mmol/kg/day.
LEARNING POINTS
In hypernatraemic dehydration with a serum sodium higher than 160 mmol/l, if urine output is more than adequate, suspect a renal tubular defect.
In hypernatraemia, even with clear a history of diarrhoea and absence of obvious renal impairment, it is worth measuring paired urine and plasma osmolalities and fractional excretion of sodium.
Absolute values of serum urea and creatinine in someone who is chronically bedridden, especially with a myopathy, may be misleading and their trends carry more meaning.
The sodium content of medications must be highlighted in someone who cannot indicate thirst; although rare, it is worth monitoring renal tubular function in someone who is neurologically impaired and is on sodium valproate.
Normal anion gap metabolic acidosis must prompt early evaluation for renal tubular acidosis.
Acknowledgments
We would like to thank Dr Anne Thomson, Dr Josep Panisello and all the medical and nursing staff who were involved with the care of our patient
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Moritz ML, Ayus JC. Isotonic maintenance fluids do not produce hypernatraemia. Arch Dis Child 2009; 94: 170. [DOI] [PubMed] [Google Scholar]
- 2.Battle DC, Hizon M, Cohen E, et al. The use of the urinary anion gap in the diagnosis of hyperchloremic metabolic acidosis. N Engl J Med 1988; 318: 594–9 [DOI] [PubMed] [Google Scholar]
- 3.Adrogue HJ, Madias NJ.Renal tubular acidosis.:Davison AM, Cameron JS, Grunfeld JP, et al., eds. Oxford textbook of clinical nephrology. Vol 2. London: Oxford University Press, 2005: chapter 5.4 [Google Scholar]
- 4.Knorr M, Schaper J, Harjes M, et al. Fanconi syndrome caused by antiepileptic therapy with valproic acid. Epilepsia 2004; 45: 868–71 [DOI] [PubMed] [Google Scholar]
- 5.Jaffe SL, Sanford M. Valproate-induced renal proximal tubular dysfunction: clinically relevant in the severely disabled epileptic population. Epilepsia 2005; 46: 599–600 [DOI] [PubMed] [Google Scholar]
- 6.Coulthard MG. Will changing maintenance intravenous fluid from 0.18% to 0.45% saline do more harm than good? Arch Dis Child 2008; 93: 335–40 [DOI] [PubMed] [Google Scholar]
- 7.Coulthard MG, Haycock GB. Distinguishing between salt poisoning and hypernatremic dehydration in children. BMJ 2003; 326: 157–60 [DOI] [PMC free article] [PubMed] [Google Scholar]