Abstract
Stiff-person syndrome (SPS) is a rare neurological condition consisting of progressive and fluctuating rigidity of the axial muscles combined with painful spasms. The pathophysiology of SPS is not fully understood, but there seems to be an autoimmune component. The use of rituximab, a chimeric monoclonal antibody targeting CD20 protein in the surface of mature B cells, for the treatment of SPS is a recent therapeutical approach showing promising results. The authors present a case report of a 41-year-old female patient diagnosed with SPS who was treated with rituximab in a public hospital in Brasília, Brazil, showing a good and safe response to the treatment so far. Our data go along with some recent articles published in the literature.
Background
This case report adds new information on a rare disease. The idea for this paper came from an article published in the Journal of Neurology, Neurosurgery and Psychiatry, showing good results using rituximab in the treatment of stiff-person syndrome (SPS).1
Case presentation
A 41-year-old female patient presented with a history of rigidity to the abdominal wall and paravertebral muscles associated with painful spasms in the lower back region, increased tonus on the shoulder and paravertebral region, and an exaggerated lumbar lordosis beginning 7 years prior to admission with a progressive course. Three months before seeking our service, she experienced worsening in the frequency and intensity of the spasms, with severe functional limitation, inability to get out of bed and having lost approximately 20 kg (44 lb) during the period. The patient underwent a thorough investigation for occult neoplasm, which was negative. SPS was proposed as a possible diagnosis and tests for anti-glutamic acid decarboxylase (GAD) antibodies were performed showing a positive result in high titres. Together with an electromyography showing continuous motor activity with normal morphology both on paravertebral and abdominal muscles, the evidences allowed us to make the diagnosis of SPS (figure 1–2).
Figure 1.
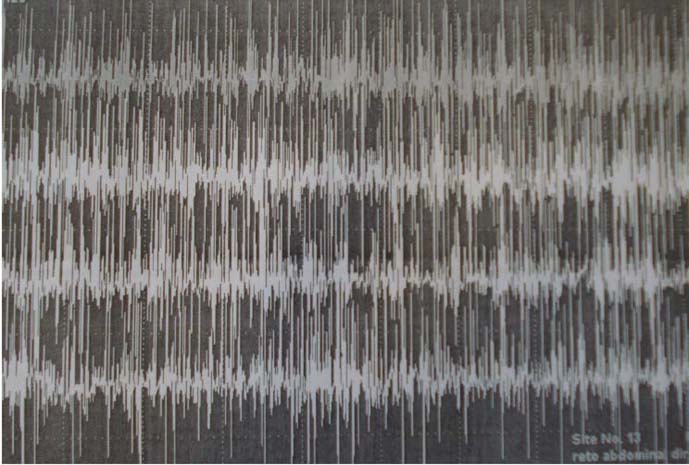
Eletroneuromyography of the right rectus abdominal muscle before treatment.
Figure 2.
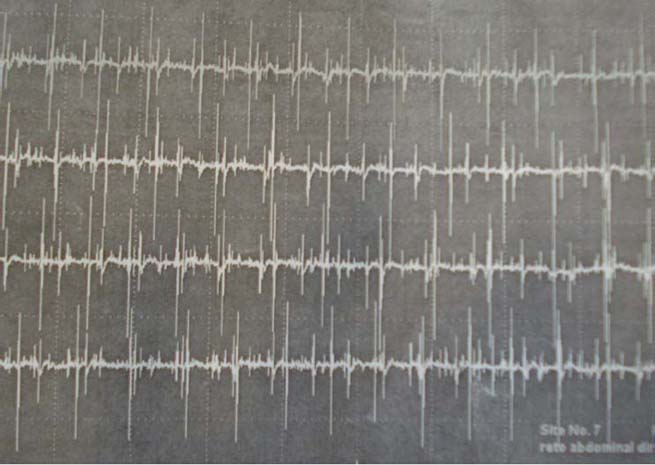
Eletroneuromyography of the right rectus abdominal muscle 16 days after the first rituximab infusion.
The patient's response to the symptomatic treatment with intravenous diazepam and application of botulinum toxin type A was moderate requiring high doses of benzodiazepine; she also did not tolerate our attempts to change the intravenous to oral medication.2
The case was extensively discussed at our unit and, although some good results have been reported with the use of plasma exchange and hyper immune-globulin infusion, we decided to try rituximab for the treatment of SPS based on recent papers showing evidence of a good and safe response. The treatment was conducted in accordance with the Declaration of Helsinki and all the procedures were carried out with the adequate understanding of the subject who read and signed an informed consent before we started it.
Investigations
Head and chest CT with contrast infusion; thyroid, abdomen, pelvis and transvaginal ultrasound; upper gastrointestinal tract endoscopy and colonoscopy were carried out. Unfortunately, the F-18-fluorodeoxyglucose positron emission tomography was not performed because we do not have access to this exam.
SPS is also associated with anti-pancreatic islet cell antibodies and anti-amphiphysin antibodies. The first one is associated with diabetes, which was ruled out in our patient, and the second one is associated with paraneoplastic syndromes. Since the investigation was negative and the financial resources of our service are limited, we did not investigate these antibodies.
The effect of the treatment has lasted for about 1 year and, so far, monotherapy with rituximab seems to be a good choice for the treatment; however it may not be enough for maintaining remission because our patient still needs to use benzodiazepines as symptomatic drugs.
Outcome and follow-up
Two days after the infusion of rituximab (dose 375 mg/m2) she started showing decrease in the muscular tonus and required a progressively smaller dose of intravenous benzodiazepine. The second infusion occurred 15 days after the first one and the next day she tolerated oral diazepam without spasms. She did not show any side effects to the medication and was dismissed 8 days after the second infusion to outpatient follow-up.
Discussion
SPS is a rare neurological disease and, although its physiopathology is not well understood, there is evidence that anti-GAD antibodies interact with the motor interneurons interfering with gamma-aminobutyric acid (GABA)-mediated inhibition leading to continuous motor activity in some muscle groups, initially affecting axial muscles, but also compromising limb muscles in some cases.1 3–5 9
SPS was originally described in 1956 by Moersch and Woltman.6 It is mostly a clinical diagnosis, facilitated by a high degree of suspicion, due to the lack of disease-specific neurological signs and laboratory tests. The electromyographic signs of simultaneous involuntary contraction of the agonist and antagonist muscle groups, although helpful when the disease is suspected, are not specific.1 7
Our patient was diagnosed based on clinical features, neurophysiological exams and serological testing for anti-GAD antibodies. Symptomatic treatment is based on GABA-enhancing medications, leading to reduction of the continuous motor activity, but the specific treatment must be targeted to the underlying cause, which is probably autoimmune.1 3–4 6 8
Rituximab is a monoclonal antibody targeting the CD20 antigens on the surface of mature B lymphocytes. After binding to these antigens, it initiates a cascade of biochemical events leading to the cell lysis. Its use has been approved for numerous diseases, including rheumatoid arthritis and non-Hodgkin B-cell lymphomas, and it has been tested for other diseases, such as neuromyelitis optica, with promising results. The use of rituximab in the treatment of SPS is a recent approach and good results have been reported.1
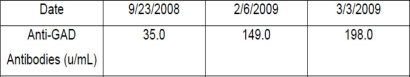
According to the available literature, a lowering in anti-GAD titres is associated with clinical improvement; however, these data were not observed in our case and, even though the patient had clinical improvement, the antibody titres continued rising (figure 3). It is possible that anti-GAD antibody titre should have no relation to the clinical presentation of the disease. This is a preliminary paper showing partial results and, therefore, a long-term follow-up is required.
Figure 3.
Serum dosage of Anti-GAD Antibodies. First infusion was 26 Jan 2009 and second infusion was 12 Feb 2009.
Learning points.
-
▶
SPS is probably an autoimmune disease.
-
▶
It is associated with anti-GAD antibodies, but its concentration seems to have no correlation to the clinical symptoms.
-
▶
Rituximab may be a promising option in its treatment.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Baker MR, Das M, Isaacs J, et al. Treatment of stiff person syndrome with rituximab. J Neurol Neurosurg Psychiatr 2005;76:999–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snow BJ, Tsui JKC, Bhatt MH, et al. Treatment of spasticity with botulinum toxin: a doubleblind study. Ann Neurol 1990;28:512–15 [DOI] [PubMed] [Google Scholar]
- 3.Rodgers-Neame N. Stiff Person Syndrome, 2009. http://emedicine.medscape.com/article/1172135-overview (accessed 10 Sep 2009)
- 4.Cabo-López I, Negueruela-López M, Garcia-Bermejo P, et al. Síndrome de la persona rígida: a propósito de un caso. Rev Neurol 2008;47:249–52 [PubMed] [Google Scholar]
- 5.Liguori R, Cordivari C, Lugaresi E, et al. Botulinum toxin A improves muscle spasms and rigidity in stiff-person syndrome. Mov Disord 1997;12:1060–3 [DOI] [PubMed] [Google Scholar]
- 6.Spitz M, Ferraz HB, Barsottini OG, et al. Progressive encephalomyelitis with rigidity: a paraneoplastic presentation of oat cell carcinoma of the lung. Case report. Arq Neuropsiquiatr 2004;62:547–9 [DOI] [PubMed] [Google Scholar]
- 7.Dalakas MC, Fujii M, Li M, et al. The clinical spectrum of anti-GAD antibody-positive patients with stiff-person syndrome. Neurology 2000;55:1531–5 [DOI] [PubMed] [Google Scholar]
- 8.Barker RA, Revesz T, Thom M, et al. Review of 23 patients affected by the stiffman syndrome: clinical subdivision into stifftrunk, stifflimb and progressive encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatr 1998;65:633–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy LM, Dalakas MC, Floeter MK. The stiff-person syndrome: an autoimmune disorder affecting neurotransmission of {gamma}-aminobutyric acid. Ann Intern Med 1999;131:522–30 [DOI] [PubMed] [Google Scholar]