Abstract
Background
Psychiatric symptoms occur frequently in the course of AD, are a frequent contributor to institutionalization, predict cognitive decline and death, and often require treatment with psychotropic medications. Previous studies investigating the association between APOE genotype and psychiatric symptomatology in AD have reported contradictory results.
Objective
To determine whether APOE genotype predicts incident psychiatric symptomatology in patients with AD.
Methods
Eighty-seven patients with AD at early stages and no psychiatric history were followed semiannually for up to 9.3 years (mean 5.5 years) for development of delusions, illusions, hallucinations, behavioral symptoms, and depression. Cox proportional hazards models were used to examine the relative risk for incident psychiatric symptomatology (outcome) in relation to APOE genotype (predictor).
Results
The presence of one ε4 allele carried a 2.5-fold risk, whereas the presence of two ε4 alleles carried a 5.6-fold risk for development of delusions. The associations remained significant even when age, ethnicity, sex, education, duration of disease, and cognitive and functional performance were controlled for. The presence of two ε4 alleles was associated with reduced risk for developing hallucinations in the adjusted analysis only. No significant associations were detected between APOE genotype and the incidence of illusions, behavioral symptoms, or depression.
Conclusion
The presence of one or more ε4 alleles is a significant predictor for the incidence of delusions in the course of AD.
Psychiatric symptoms occur frequently in the course of AD. The prevalence of delusions over the course of the illness is estimated at up to 75%,1 of hallucinations to 50%,1 and of psychotic symptoms in general to 75%.1 Behavioral disturbances like wandering, agitation, or physical aggression have been noted in 52% to 71%,2 whereas mood disorders in general are also commonly seen, with frequencies of 27% to 69%.2,3 Major depression, in particular, may be seen in up to 30% of patients with AD.2,3
Psychiatric symptoms are a major cause of anxiety and concern to both patients and caregivers and a frequent contributor to institutionalization.4,5 They often require treatment with psychotropic medications, the use of which is associated with increased side effects in the elderly.6 In addition, the presence of psychiatric symptoms in AD has been shown to be a predictor of faster cognitive decline and death.7-9
Some investigators have reported significant associations between APOE genotype and various psychiatric symptoms.3,10-15 However, the majority of studies failed to demonstrate a significant relation.16-27 All these studies were cross-sectional. The emergence of specific psychiatric manifestations is associated with the severity of cognitive impairment,2,20 which in turn is largely a reflection of duration of illness. Psychotic symptoms, agitation, and aggression are considered to be uncommon in the early stages of the disease and become more frequent as the disease progresses.2,20,28 Therefore, examining only the prevalence of these symptoms without taking into account “how far” the patient is into the disease course is an important limitation of previous studies. The use of patients with AD with variable duration of illness (and therefore of different stages of the disease) in previous cross-sectional studies has provided useful information but has not accurately recorded the time to development of psychiatric symptomatology.
We assessed the presence and nature of psychopathologic features every 6 months in a prospectively followed cohort of patients with early possible and probable AD. We determined whether APOE geno-type was associated with differential risk for development of these psychiatric manifestations.
Methods
Subjects
Subjects were drawn from the Predictors Study.29,30 Patients met Diagnostic and Statistical Manual (3rd rev. ed.) criteria for primary degenerative dementia of the Alzheimer type31 and National Institute of Neurologic Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria for probable AD.32 Enrollment required a modified Mini-Mental State Examination (mMMSE) score of ≥30 (maximum mMMSE score, 57), which is approximately equivalent to a score of ≥16 on the original MMSE.33-35
For the purposes of the present analyses, we excluded patients with any history of either psychiatric hospitalization or use of medications for psychiatric problems at any time before the first evaluation. Although elimination of patients with AD based on premorbid psychiatric history might result in a subset of patients with lower frequency of depression that emerges after the onset of AD,36 it simultaneously served the purpose of excluding subjects with psychopathologic symptoms emerging after the onset of AD. The resulting psychiatric symptom-free population was appropriate for evaluation not only of the frequency but also of the time until occurrence of incident psychiatric symptomatology (as required by survival analyses models).
Evaluation
At the initial visit, various demographic (age, ethnicity, sex, education, and so on) and disease severity features were assessed. Duration of illness as estimated by the clinician based on interviews with the patient and the informant was also recorded. The clinicians determined whether the patient was ever hospitalized or medications ever prescribed for psychiatric problems. Neurologic, psychiatric, and mental status examinations were conducted at study entry and at 6-month intervals thereafter. Cognitive function was examined using the mMMSE.33-35 Functional capacity was assessed using the Blessed Dementia Rating Scale (BDRS).37
APOE
APOE studies were not an original component of the Predictors Study (because when data collection was initiated, APOE testing was not available), but beginning in the sixth year of the study, available subjects were approached to contribute blood samples for analyses. The pattern of each subject's APOE isoforms was determined using the method of Hixson and Powers.38 In brief, white blood cells were isolated from fresh blood samples after centrifugation at 2,000 rpm for 20 minutes at 4 °C, and DNA was prepared. The DNA was amplified by PCR using APOE-specific oligonucleotide primers and Taq polymerase. The APOE PCR products were then digested with 5 units of HhaI enzyme at 37 °C for 4 hours. The digest was electrophoresed on a 12% nondenaturing polyacrylamide gel for 3 hours at a constant current of 10 mA. The gels were treated with ethidium bromide for 10 to 15 minutes, and the DNA fragments were visualized by ultraviolet illumination. Fragments of DNA of known size were used as markers.
For the purpose of the analysis, patients were categorized into three groups: those with two ε4 alleles (ε4/ε4), those with one (ε3/ε4 or ε2/ε4), and those without any (ε3/ε3 or ε2/ε2 or ε3/ε2). The resulting three-level categorical variable was introduced as the predictor variable in Cox proportional hazards models, with the group without any ε4 allele being considered the reference.
Outcomes
The following outcomes were considered. A trained research technician administered the Columbia University Scale for Psychopathology in Alzheimer's Disease (CUSPAD)39 to the informant at the initial examination and at 6-month intervals thereafter. The bulk of informants were spouses (70.1%) or adult children (21.8%). The informant rarely changed during follow-up. Inter-rater reliability for the major psychiatric symptom categories between the principal scale developer and a research technician (trained by the principal scale developer), whether concurrently rating a single interview (k = 0.74 to 1.00) or conducting separate interviews (k = 0.53 to 0.73), has been reported.39
Most CUSPAD items are scored dichotomously (i.e., present or absent). For delusions, the categories were general (strange ideas or unusual beliefs), paranoid (people are stealing things, or unfaithful wife/husband, or unfounded suspicions), abandonment (accused caregiver of plotting to leave him/her), somatic (false belief that the patient has cancer or other physical illness), misidentification (false belief that people are in the house when nobody is there, or that someone else is in the mirror, or that spouse/caregiver is an impostor, or that the patient's house is not his/her home, or that the characters on television are real), and a miscellaneous category. A patient was considered to have delusions if he or she had at least one of the above types of delusions. Hallucinations (auditory, visual, tactile, and olfactory) and illusions were also recorded. Patients were considered to have hallucinations if they had hallucinations in any of the four sensory modalities.
The items for depression were depressed mood (sad, depressed, blue, down in the dumps), difficulty sleeping, and change in appetite. Depression was considered in two different ways. Initially, patients were considered depressed if they had depressed mood only, regardless of responses on the sleep and appetite items. The analyses were repeated using as the definition of depression either depressed mood and difficulty sleeping or depressed mood and appetite changes. Because the results were similar, the analyses reported use of the first definition of depression.
The five items for behavioral disturbance were wandering away from home, verbal outbursts, physical threats or violence, agitation or restlessness, and sundowning (more confusion at night or during the evening compared with daytime). If a patient manifested any of the five behavioral disturbances, he or she was considered to have behavioral symptoms.
Statistical analysis
The χ2 test and analysis of variance were used to examine associations between the APOE genotype and demographic characteristics or clinical features.
Cox proportional hazards analyses40 used APOE geno-type as the predictor variable and the psychiatric symptoms as the outcome variables. Duration (in years) between the initial visit and either development of psychiatric symptoms or last evaluation without psychiatric symptoms was used as the timing variable. Patients with the outcome psychiatric symptoms at first evaluation were not included in the Cox analyses. Initial Cox models considered only the association between APOE and the incidence of psychiatric symptoms. In subsequent analyses, age, ethnicity, sex, education, disease duration, mMMSE score, and BDRS score (as recorded at initial evaluation) were introduced as covariates.
Results
Demographic/clinical features and APOE
Of the 252 patients with AD in the Predictors Study, 50 had history of either hospitalization for psychiatric reasons or treatment with medications for psychiatric reasons, and 4 subjects were missing these data. These patients were excluded from subsequent analyses. The subjects were followed for up to 9.3 years (mean 5.5 years, SD 2.3 years).
APOE genotypes were available for 87 of the remaining 198 subjects. APOE genotype distribution was as follows: ε4/ε4, 13 (14.9%); ε4/ε3, 32 (36.8%); ε4/ε2, 3 (3.4%); ε3/ε2, 5 (5.7%); and ε3/ε3, 34 (39.1%) (a distribution similar to that in other series of AD populations).41,42 Those subjects for whom APOE genotype was not available, in comparison with those included in this study for whom APOE was available, were more likely to be female (65.2% compared with 34.8%; p ≤ 0.002) and were more likely to manifest behavioral symptomatology (53.3% compared with 46.7%; p ≤ 0.012). The two groups did not differ significantly in ethnic distribution, mean age, mean duration of disease, mean education, mean mMMSE scores at initial evaluation, mean BDRS scores at initial evaluation, or manifestation of any other psychiatric symptomatology.
Demographic, neuropsychologic, and clinical information at first evaluation as well as APOE genotype grouping according to number of ε4 alleles are presented in table 1. Sex (p ≤ 0.79), age (p ≤ 0.87), duration of disease (p ≤ 0.91), years of education (p ≤ 0.92), mMMSE score (p ≤ 0.18), and BDRS score (p ≤ 0.10) were similar across the subject groups with two or one or no ε4 allele. Because 89.7% of the subjects were white, no further analyses examining the distribution of APOE genotype among ethnic groups were deemed necessary.
Table 1.
Demographic, APOE, clinical, and neuropsychologic information at first evaluation for all subjects, n = 87
| Data | n |
|---|---|
| Men (%) | 47 (54) |
| Women (%) | 40 (46) |
| Ethnicity | |
| white (%) | 78 (89.7) |
| black (%) | 4 (4.6) |
| hispanic (%) | 5 (5.7) |
| Mean age (SD), y | 70.7 (7.8) |
| Mean duration of disease (SD), y | 3.9 (2.1) |
| Mean education (SD), y | 13.6 (3.7) |
| Mean mMMSE (SD) | 37.8 (6.2) |
| Mean BDRS (SD) | 7.7 (3.2) |
| Two ε4 alleles (%) | 13 (14.9) |
| One ε4 allele (%) | 35 (40.2) |
| No ε4 allele (%) | 39 (44.8) |
mMMSE = modified Mini-Mental State Examination; BDRS = Blessed Dementia Rating Scale.
Numbers of patients without psychiatric symptomatology at first evaluation who were used in each Cox model are shown in table 2. In addition, table 2 presents numbers and percentages of subjects who developed incident psychiatric symptomatology at follow-up visits overall and by APOE genotype.
Table 2.
Numbers and percentages of patients manifesting incident psychiatric symptoms at any follow-up visit, overall and within each APOE genotype group
| No. of subjects overall |
No. (%) of subjects manifesting symptom |
|||||
|---|---|---|---|---|---|---|
| Incident psychiatric symptoms | No symptom at first visit | Manifesting symptom at follow-up | Two ε4 alleles | One ε4 allele | No ε4 allele | p Value |
| Delusions | 58 | 49 | 8 (100) | 21 (95.5) | 20 (71.4) | 0.028 |
| Hallucinations | 77 | 35 | 2 (16.7) | 18 (56.3) | 15 (45.5) | 0.063 |
| Illusions | 81 | 17 | 4 (30.8) | 5 (15.6) | 8 (22.2) | 0.512 |
| Depression | 49 | 36 | 3 (42.9) | 15 (78.9) | 18 (78.3) | 0.140 |
| Behavioral | 38 | 36 | 6 (100) | 10 (100) | 20 (90.9) | 0.464 |
p Values are derived from χ2 analyses of the following two variables: development of psychiatric symptomatology and APOE genotype group.
Cox analyses
Survival analyses have the advantage of combining information about both frequency of events (as in table 2) and time of occurrence of events. Patients without any ε4 alleles constituted the reference group in all subsequent Cox analyses, the results of which are presented in table 3.
Table 3.
Cox proportional hazards models predicting psychiatric symptomatology by APOE genotype
| Type of analysis | Psychiatric symptom | RR (95% CI) when presence of one ε4 allele | RR (95% CI) when presence of two ε4 alleles |
|---|---|---|---|
| Simple model | Delusions | 2.5 (1.3–4.8)* | 5.6 (2.2–14.0)* |
| Hallucinations | 1.2 (0.6–2.5) | 0.3 (0.1–1.3) | |
| Illusions | 0.6 (0.2–1.7) | 1.4 (0.4–4.7) | |
| Depression | 1.0 (0.5–2.0) | 0.3 (0.1–1.1) | |
| Behavioral | 1.3 (0.6–2.8) | 0.8 (0.3–2.1) | |
| Adjusted model | Delusions | 3.0 (1.4–6.3)* | 11.7 (3.8–36.0)* |
| Hallucinations | 0.9 (0.4–1.9) | 0.2 (0.0–0.9)* | |
| Illusions | 0.5 (0.1–1.7) | 1.2 (0.3–5.2) | |
| Depression | 2.4 (1.0–5.8) | 0.4 (0.1–1.6) | |
| Behavioral | 1.4 (0.5–3.8) | 1.5 (0.6–4.3) |
Results for both the simple model (APOE genotype being the predictor without inclusion of other covariates) and the adjusted model (age, sex, ethnicity, disease duration, modified Mini-Mental State Examination, and Blessed Dementia Rating Scale score included as covariates) are presented.
Significant results.
RR = relative risk.
Simple model
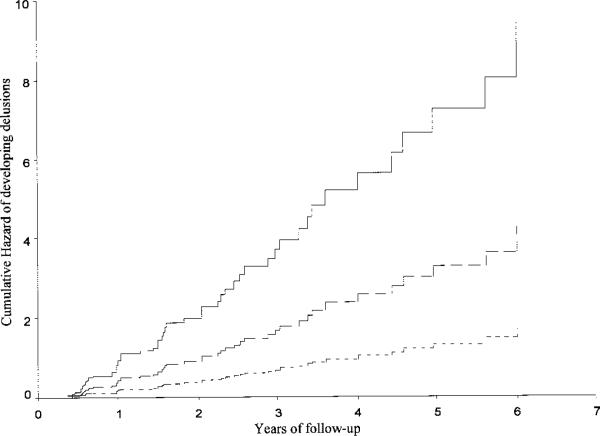
The presence of one ε4 allele was associated with a relative risk (RR) of 2.5 (95% CI 1.3 to 4.8) for subsequent development of delusions. Cumulative hazard curves are presented in the figure. There was no significant association between APOE status and either hallucinations, illusions, depression, or behavioral symptoms.
Figure.
Cumulative hazard curves for developing delusions according to APOE genotype. The time axis is years from first evaluation until development of delusions (or last evaluation). Solid line = subjects with two ε4 alleles; long dashed line = subjects with one ε4 allele; short dashed line = subjects with no ε4 allele.
Adjusted model
In these analyses, age, ethnicity, sex, education, disease duration, mMMSE score, and BDRS score were simultaneously introduced as covariates in Cox models. The presence of one ε4 allele was associated with an RR of 3.0 (95% CI 1.4 to 6.3) for development of delusions. When two ε4 alleles were present, there was an 11.7 RR (95% CI 3.8 to 36.0) for development of delusions. Analyses for the other psychiatric symptoms did not reveal any significant associations with the APOE genotype, except for a negative correlation between the presence of two ε4 alleles and the development of hallucinations (RR 0.2; 95% CI 0.0 to 0.9).
Discussion
Many studies failed to detect any association between APOE genotype and either delusions or psychosis in patients with AD.12,16,17,19-24,26 On the contrary, other studies11,15 have reported an increased frequency of psychosis in ε4 carriers. Similarly, it has been shown that ε4 was associated with psychosis (in particular, at the advanced stages of the disease),14 and an increased frequency of hallucinations and psychotic disturbances has been noted in patients with AD with the ε4 allele.13
Our key finding was a strong and dose-related association between the APOE genotype and the incidence of delusions. We also noted that the presence of two ε4 alleles was associated with a decreased risk for developing hallucinations (RR 0.2; 95% CI 0 to 0.9; p < 0.04) in the adjusted model only. This was not seen with one ε4 allele in the same model (RR 0.9; 95% CI 0.4 to 1.9; p < 0.71). In addition, this effect was not seen for one or two ε4 alleles in the unadjusted model. Given this weak association, the implications of this finding are unclear. One possible explanation is that treatment during follow-up with either antipsychotic or anticholinesterase medications in subjects with one or two ε4 alleles (who might have received these treatments because of more delusions) might have resulted in suppression of hallucinations and therefore artificially decreased incidence. To test this idea, we excluded subjects who received any of the above medications and repeated the analyses. The protective effect was no longer present (simple model—one ε4: RR 1.7, 95% CI 0.72 to 4.02, two ε4: RR 0.5, 95% CI 0.11 to 2.28; adjusted model–one ε4: RR 1.3, 95% CI 0.45 to 3.53, two ε4: RR 0.5, 95% CI 0.9 to 2.72), supporting this possibility. The results for delusions remained unchanged. Alternatively, there might be a real dissociation between the incidence of delusions and hallucinations. This would not be surprising given that we observe this dissociation in AD (where delusions are more common than hallucinations) and in diffuse Lewy body dementia (where hallucinations are more common than delusions). It may suggest differential pathophysiology or pathoanatomic localization underlying the development of these symptoms.
With regard to other psychiatric manifestations in AD, some studies did not detect any association between APOE genotype and depressive symptoms in AD samples.18,19,23,25,26 APOE genotype was not associated with any changes in depression scores in healthy elderly community residents.27 However, it has been reported that patients with AD with ε3/ε4 genotype were more likely to be depressed11 and that a positive correlation exists between the ε4 allele and mood disturbances.13 Unlike the previous studies, an increased risk for depression in carriers of the ε2 (rather than the ε4) allele12 and a protective role for the ε4 allele for depressive symptoms15 have been published. The current study failed to detect any association between APOE genotype and depressive symptomatology.
Behavioral disturbances have also been investigated in relation to APOE genotype. Although agitation was more common in ε4 carriers in one study,10 in a subsequent study, agitation and disorientation were more common in ε4/ε4 carriers, whereas anxiety and sleep disorders were more common in ε3/ε4 carriers.3 In another report, it was noted that the presence of the ε4 allele was associated with increased combativeness, agitation, wandering, and confusion.13 We detected no association between the APOE genotype and incidence of behavioral disturbances in our cohort.
Explanations for the variable findings in previous studies include differences in patient populations (some of the studies included subjects with Lewy body disease), differences in the assessment of psychiatric symptomatology, and possible lack of statistical power to detect associations in the negative studies. In addition, many of the above studies did not control for potential confounding variables. Most important, all of the previous studies were cross-sectional, whereas the current study evaluated risk for incident symptoms in a prospectively followed cohort.
The current analyses are performed on a well-characterized sample of patients with probable AD who underwent a rigorous diagnostic process in AD research centers. Patients with a diagnosis of diffuse Lewy body disease or other dementias were not included. A widely used scale for psychopathology with well-documented reliability and validity was used. Additional strengths of the current cohort include enrollment of subjects who were at a relatively early stage of the disease and the long follow-up with semiannual recording of psychopathology. Thus, we have reliably recorded the progression of AD from its early stages, and we have accurately assessed the incidence of psychiatric symptoms.
We also tried to control for all potential confounders. Low educational attainment has been associated with high risk for developing AD43 and psychosis in AD.20 Similarly, female gender has been considered a risk factor in AD and has been related to APOE status.20 We included both education and sex as covariates in our analyses. Because severity of AD might be associated with the risk for psychopathologic manifestations,2,20 we controlled for cognitive (mMMSE) and functional (BDRS) impairment as well as age and disease duration.
Different APOE genotypes seem to confer different risks for developing AD (and therefore possible differential biologic effects) among different ethnic groups.42,44 Given the fact that the ethnic distribution of this AD population was heavily weighted for white persons, with few blacks or Hispanics, the results of the current study might not be generalizable to all ethnic groups. In addition, the population was derived from major university-based AD referral centers, and it might not be generalizable to the population of patients with AD in the community. Finally, it is plausible that the modest sample size of 87 subjects might have resulted in inadequate power to detect associations between the APOE genotype and illusions, hallucinations, depression, and behavioral symptoms.
The clinical heterogeneity observed in AD45 could derive from underlying genetic variability. For example, the presence of the ε4 allele has been associated with earlier age at disease onset.41 The relation of APOE genotype to extrapyramidal signs in AD has also been investigated. Psychotic manifestations and extrapyramidal signs are well-characterized predictors of rate of progression and outcomes in AD.7-9 The presence of the ε4 allele was associated with increased risk for psychosis in the current study but has been inversely associated with the development of extrapyramidal signs in previous reports.46 Finally, the association between the APOE genotype and rate of cognitive decline or outcomes in AD has been controversial.7,41,46,47
In terms of neuropathologic correlates, patients with AD with the ε4 allele have increased β-amyloid burden.48,49 Psychiatric symptomatology has been associated with more severe neuropathologic changes.50,51 It is therefore plausible that the increased incidence of delusions in subjects with ε4 alleles could be a manifestation of more severe underlying pathology.
The ε4 allele has been associated with more profound deficits in cholinergic neurons, in particular in the frontal lobes,52,53 whereas the development of psychiatric symptoms in AD appears to be related to specific neurotransmitter imbalances, notably acetylcholine.54 Consequently, the presence of the ε4 allele could favor a preferential pathologic involvement of the cholinergic system, which in turn might result in more frequent psychiatric manifestations.
Psychotic manifestations in AD have been associated with pathology in the temporal lobe and hippocampus.50,55 SPECT evidence has suggested that delusions in AD may be associated with greater temporal lobe hypoperfusion.56 At the same time, SPECT and MRI studies have shown greater atrophy in medial temporal structures and more severe loss in hippocampal volumes in patients with AD carrying the ε4 allele.57,58 It is conceivable that the detected association between APOE genotype and delusions might reflect neuropathology more heavily concentrated in the temporal lobe. Despite these speculations, the pathophysiologic explanation of the association between the APOE genotype and development of delusions in AD is not clear.
The observed association between the APOE geno-type and development of delusions is important, given the medical and social impact of psychiatric symptomatology in AD, its prognostic value for future outcomes, and the responsiveness of psychosis in AD to treatment.59-62
Acknowledgments
Supported by federal grants AG07370 and RR00645 and the Taub Institute for Research in Alzheimer's Disease and the Aging Brain.
References
- 1.Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer type. Biol Psychiatry. 1989;25:39–48. doi: 10.1016/0006-3223(89)90145-5. [DOI] [PubMed] [Google Scholar]
- 2.Devanand DP, Jacobs DM, Tang MX, et al. The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry. 1997;54:257–263. doi: 10.1001/archpsyc.1997.01830150083012. [DOI] [PubMed] [Google Scholar]
- 3.Cacabelos R, Rodriguez B, Carrera C, Beyer K, Lao JI, Sellers MA. Behavioral changes associated with different apolipoprotein E genotypes in dementia. Alzheimer Dis. 1997;11:S27–S34. [PubMed] [Google Scholar]
- 4.Steele C, Rovner B, Chase GA, Folstein M. Psychiatric symptoms and nursing home placement of patients with Alzheimer's disease. Am J Psychiatry. 1990;147:1049–1051. doi: 10.1176/ajp.147.8.1049. [DOI] [PubMed] [Google Scholar]
- 5.Rabins PV, Mace NL, Lucas MJ. The impact of dementia on the family. JAMA. 1982;248:333–335. [PubMed] [Google Scholar]
- 6.Byerly MJ, Weber MT, Brooks DL, Snow LR, Worley MA, Lescouflair E. Antipsychotic medications and the elderly: effects on cognition and implications for use. Drugs Aging. 2001;18:45–61. doi: 10.2165/00002512-200118010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Stern Y, Tang MX, Albert MS, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA. 1997;277:806–812. [PubMed] [Google Scholar]
- 8.Stern Y, Mayeux R, Sano M, Hauser WA, Bush T. Predictors of disease course in patients with probable Alzheimer's disease. Neurology. 1987;37:1649–1653. doi: 10.1212/wnl.37.10.1649. [DOI] [PubMed] [Google Scholar]
- 9.Stern Y, Albert M, Brandt J, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer's disease: prospective analyses from the Predictors Study. Neurology. 1994;44:2300–2307. doi: 10.1212/wnl.44.12.2300. [DOI] [PubMed] [Google Scholar]
- 10.Cacabelos R, Rodriguez B, Carrera C, et al. APOE-related frequency of cognitive and noncognitive symptoms in dementia. Methods Find Exp Clin Pharmacol. 1996;18:693–706. [PubMed] [Google Scholar]
- 11.Ramachandran G, Marder K, Tang M, et al. A preliminary study of apolipoprotein E genotype and psychiatric manifestations of Alzheimer's disease. Neurology. 1996;47:256–259. doi: 10.1212/wnl.47.1.256. [DOI] [PubMed] [Google Scholar]
- 12.Holmes C, Levy R, McLoughlin DM, Powell JF, Lovestone S. Apolipoprotein E: non-cognitive symptoms and cognitive decline in late onset Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1996;61:580–583. doi: 10.1136/jnnp.61.6.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy GM, Taylor J, Tinklenberg JR, Yesavage JA. The apolipoprotein E epsilon 4 allele is associated with increased behavioral disturbance in Alzheimer's disease. Am J Geriatr Psychiatry. 1997;5:88–89. doi: 10.1097/00019442-199700510-00012. [DOI] [PubMed] [Google Scholar]
- 14.Harwood DG, Barker WW, Ownby RL, St George-Hyslop P, Duara R. Apolipoprotein-E (APO-E) genotype and symptoms of psychosis in Alzheimer's disease. Am J Geriatr Psychiatry. 1999;7:119–123. [PubMed] [Google Scholar]
- 15.Ballard C, Massey H, Lamb H, Morris C. Apolipoprotein E: non-cognitive symptoms and cognitive decline in late onset Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1997;63:273. doi: 10.1136/jnnp.63.2.273b. [DOI] [PubMed] [Google Scholar]
- 16.Lehtovirta M, Soininen H, Helisalmi S, et al. Clinical and neuropsychological characteristics in familial and sporadic Alzheimer's disease: relation to apolipoprotein E polymorphism. Neurology. 1996;46:413–419. doi: 10.1212/wnl.46.2.413. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Alberola R, Gilchirst D, Barker WW, Geroge-Hyslop PS, Duara R. Analysis of phenotype in Alzheimer's disease (AD) associated with apolipoprotein E (APOE) alleles. Neurology. 1994;44(suppl 2):A207. [Google Scholar]
- 18.Forsell Y, Corder EH, Basun H, Lannfelt L, Viitanen M, Winblad B. Depression and dementia in relation to apolipoprotein E polymorphism in a population sample age 75+. Biol Psychiatry. 1997;42:898–903. doi: 10.1016/S0006-3223(96)00468-4. [DOI] [PubMed] [Google Scholar]
- 19.Lyketsos CG, Baker L, Warren A, et al. Depression, delusions, and hallucinations in Alzheimer's disease: no relationship to apolipoprotein E genotype. J Neuropsychiatry Clin Neurosci. 1997;9:64–67. doi: 10.1176/jnp.9.1.64. [DOI] [PubMed] [Google Scholar]
- 20.Hirono N, Mori E, Yasuda M, et al. Factors associated with psychotic symptoms in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1998;64:648–652. doi: 10.1136/jnnp.64.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez OL, Kamboh MI, Becker JT, Kaufer DI, DeKosky ST. The apolipoprotein E epsilon 4 allele is not associated with psychiatric symptoms or extrapyramidal signs in probable Alzheimer's disease. Neurology. 1997;49:794–797. doi: 10.1212/wnl.49.3.794. [DOI] [PubMed] [Google Scholar]
- 22.Forsell Y, Basun H, Corder EH, Lannfelt L, Winblad B. Psychotic symptoms and apolipoprotein E genotypes in an elderly population. Biol Psychiatry. 1998;44:139–140. doi: 10.1016/s0006-3223(97)00352-1. [DOI] [PubMed] [Google Scholar]
- 23.Levy ML, Cummings JL, Fairbanks LA, Sultzer DL, Small GW. Apolipoprotein E genotype and noncognitive symptoms in Alzheimer's disease. Biol Psychiatry. 1999;45:422–425. doi: 10.1016/s0006-3223(98)00041-9. [DOI] [PubMed] [Google Scholar]
- 24.Hirono N, Mori E, Yasuda M, et al. Lack of effect of apolipoprotein E E4 allele on neuropsychiatric manifestations in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 1999;11:66–70. doi: 10.1176/jnp.11.1.66. [DOI] [PubMed] [Google Scholar]
- 25.Cantillon M, Harwood D, Barker W, et al. No association between apolipoprotein E genotype and late-onset depression in Alzheimer's disease. Biol Psychiatry. 1997;41:246–248. doi: 10.1016/s0006-3223(96)00422-2. [DOI] [PubMed] [Google Scholar]
- 26.Weiner MF, Vega G, Risser RC, et al. Apolipoprotein E epsilon 4, other risk factors, and course of Alzheimer's disease. Biol Psychiatry. 1999;45:633–638. doi: 10.1016/s0006-3223(98)00222-4. [DOI] [PubMed] [Google Scholar]
- 27.Mauricio M, O'Hara R, Yesvage JA, et al. A longitudinal study of apolipoprotein-E genotype and depressive symptoms in community-dwelling older adults. Am J Geriatr Psychiatry. 2000;8:196–200. [PubMed] [Google Scholar]
- 28.Chen JY, Stern Y, Sano M, Mayeux R. Cumulative risks of developing extrapyramidal signs, psychosis, or myoclonus in the course of Alzheimer's disease. Arch Neurol. 1991;48:1141–1143. doi: 10.1001/archneur.1991.00530230049020. [DOI] [PubMed] [Google Scholar]
- 29.Stern Y, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “Predictors Study”). I. Study design, cohort description, and intersite comparisons. Alzheimer Dis. 1993;7:3–21. doi: 10.1097/00002093-199307010-00002. [DOI] [PubMed] [Google Scholar]
- 30.Richards M, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “Predictors Study”). II. Neurological, psychiatric, and demographic influences on baseline measures of disease severity. Alzheimer Dis. 1993;7:22–32. doi: 10.1097/00002093-199307010-00003. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Press; Washington, DC: 1994. Revised. [Google Scholar]
- 32.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Mayeux R, Stern Y, Rosen J, Leventhal J. Depression, intellectual impairment, and Parkinson disease. Neurology. 1981;31:645–650. doi: 10.1212/wnl.31.6.645. [DOI] [PubMed] [Google Scholar]
- 35.Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-Mental State Examination: validity and reliability. Neurology. 1987;37(suppl 1):179. [Google Scholar]
- 36.Zubenco GS, Rifai AH, Mulsant BH, Sweet RA, Pasternak RE. Premorbid history of major depression and the depressive syndrome of Alzheimer's disease. Am J Geriatr Psychiatry. 1996;4:85–90. doi: 10.1097/00019442-199624410-00010. [DOI] [PubMed] [Google Scholar]
- 37.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 38.Hixson JE, Powers PK. Restriction isotyping of human apolipoprotein A-IV: rapid typing of known isoforms and detection of a new isoform that deletes a conserved repeat. J Lipid Res. 1991;32:1529–1535. [PubMed] [Google Scholar]
- 39.Devanand DP, Miller L, Richards M, et al. The Columbia University Scale for Psychopathology in Alzheimer's disease. Arch Neurol. 1992;49:371–376. doi: 10.1001/archneur.1992.00530280051022. [DOI] [PubMed] [Google Scholar]
- 40.Lawless J. Statistical model and methods for lifetime data. Wiley; New York: 1982. [Google Scholar]
- 41.Gomez-Isla T, West HL, Rebeck GW, et al. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer's disease. Ann Neurol. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- 42.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 43.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 44.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 45.Mayeux R, Stern Y, Spanton S. Heterogeneity in dementia of the Alzheimer type: evidence of subgroups. Neurology. 1985;35:453–461. doi: 10.1212/wnl.35.4.453. [DOI] [PubMed] [Google Scholar]
- 46.Stern Y, Brandt J, Albert M, et al. The absence of an apolipoprotein epsilon4 allele is associated with a more aggressive form of Alzheimer's disease. Ann Neurol. 1997;41:615–620. doi: 10.1002/ana.410410510. [DOI] [PubMed] [Google Scholar]
- 47.Corder EH, Saunders AM, Strittmatter WJ, et al. Apolipoprotein E, survival in Alzheimer's disease patients, and the competing risks of death and Alzheimer's disease. Neurology. 1995;45:1323–1328. doi: 10.1212/wnl.45.7.1323. [DOI] [PubMed] [Google Scholar]
- 48.Polvikoski T, Sulkava R, Haltia M, et al. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. 1995;333:1242–1247. doi: 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- 49.Schmechel DE, Saunders AM, Strittmatter WJ, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zubenko GS, Moossy J, Martinez AJ, et al. Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol. 1991;48:619–624. doi: 10.1001/archneur.1991.00530180075020. [DOI] [PubMed] [Google Scholar]
- 51.Zubenko GS, Moossy J. Major depression in primary dementia. Clinical and neuropathologic correlates. Arch Neurol. 1988;45:1182–1186. doi: 10.1001/archneur.1988.00520350020008. [DOI] [PubMed] [Google Scholar]
- 52.Soininen H, Kosunen O, Helisalmi S, et al. A severe loss of choline acetyltransferase in the frontal cortex of Alzheimer patients carrying apolipoprotein epsilon 4 allele. Neurosci Lett. 1995;187:79–82. doi: 10.1016/0304-3940(95)11343-6. [DOI] [PubMed] [Google Scholar]
- 53.Soininen HS, Riekkinen PJ., Sr Apolipoprotein E, memory and Alzheimer's disease. Trends Neurosci. 1996;19:224–228. doi: 10.1016/0166-2236(96)10027-8. [DOI] [PubMed] [Google Scholar]
- 54.Cummings JL, Kaufer D. Neuropsychiatric aspects of Alzheimer's disease: the cholinergic hypothesis revisited. Neurology. 1996;47:876–883. doi: 10.1212/wnl.47.4.876. [DOI] [PubMed] [Google Scholar]
- 55.Forstl H, Burns A, Levy R, Cairns N. Neuropathological correlates of psychotic phenomena in confirmed Alzheimer's disease. Br J Psychiatry. 1994;165:53–59. doi: 10.1192/bjp.165.1.53. [DOI] [PubMed] [Google Scholar]
- 56.Starkstein SE, Vazquez S, Petracca G, et al. A SPECT study of delusions in Alzheimer's disease. Neurology. 1994;44:2055–2059. doi: 10.1212/wnl.44.11.2055. [DOI] [PubMed] [Google Scholar]
- 57.Lehtovirta M, Laakso MP, Soininen H, et al. Volumes of hippocampus, amygdala and frontal lobe in Alzheimer patients with different apolipoprotein E genotypes. Neuroscience. 1995;67:65–72. doi: 10.1016/0306-4522(95)00014-a. [DOI] [PubMed] [Google Scholar]
- 58.Lehtovirta M, Soininen H, Laakso MP, et al. SPECT and MRI analysis in Alzheimer's disease: relation to apolipoprotein E epsilon 4 allele. J Neurol Neurosurg Psychiatry. 1996;60:644–649. doi: 10.1136/jnnp.60.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Deyn PP, Rabheru K, Rasmussen A, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology. 1999;53:946–955. doi: 10.1212/wnl.53.5.946. [DOI] [PubMed] [Google Scholar]
- 60.Devanand DP, Marder K, Michaels KS, et al. A randomized, placebo-controlled dose-comparison trial of haloperidol for psychosis and disruptive behaviors in Alzheimer's disease. Am J Psychiatry. 1998;155:1512–1520. doi: 10.1176/ajp.155.11.1512. [DOI] [PubMed] [Google Scholar]
- 61.Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry. 1999;60:107–115. doi: 10.4088/jcp.v60n0207. [DOI] [PubMed] [Google Scholar]
- 62.Street JS, Clark WS, Gannon KS, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch Gen Psychiatry. 2000;57:968–976. doi: 10.1001/archpsyc.57.10.968. [DOI] [PubMed] [Google Scholar]