Abstract
Reversible posterior leucoencephalopathy syndrome (RPLS) is a potentially fatal but reversible clinico-radiological syndrome with symptoms of headache, altered mental functioning, visual changes and seizures in association with typical posterior cerebral white matter lesions. RPLS is associated with the use of cytotoxic drugs, usually in combination with high blood pressure. We report a case of RPLS that we believe is associated with bortezomib, a proteasome inhibitor with proapoptotic and antiangiogenic properties approved for the treatment of relapsed multiple myeloma, and speculate about the possible mechanisms leading to RPLS. Clinicians should be aware of the potential association between RPLS and bortezomib because timely recognition and appropriate treatment are important in the prevention of irreversible neurological complications.
Background
Molecularly targeted cytotoxic agents with antiangiogenic and proapoptotic properties are increasingly being used for the treatment of haematological malignancies and solid tumours. Since the introduction of bevacizumab and sorafenib, several case reports have reported on the association between these antiangiogenic agents and reversible posterior leucoencephalopathy syndrome (RPLS).1–4 We now report on a possible association between bortezomib, a proteasome inhibitor with antiangiogenic and proapoptotic properties, and RPLS. Clinicians should be aware of the potential association between RPLS and bortezomib. This is of particular interest as we expect agents such as bortezomib to have a mounting range of indications in the near future.
Case presentation
A 62-year-old woman was diagnosed with multiple myeloma in 2003. She achieved a complete remission after chemotherapy and tandem autologous allogeneic stem cell transplantation in 2004. This was complicated by mesangiocapillary glomerulonephritis possibly caused by graft versus host disease. In October 2005 she developed progressive oedema which was unresponsive to furosemide, so she was switch to lisinopril 5 mg once daily. In August 2007 the multiple myeloma relapsed with an extramedullary plasmacytoma in the left adrenal gland, which was treated with radiation therapy. At that time her blood pressure was normal.
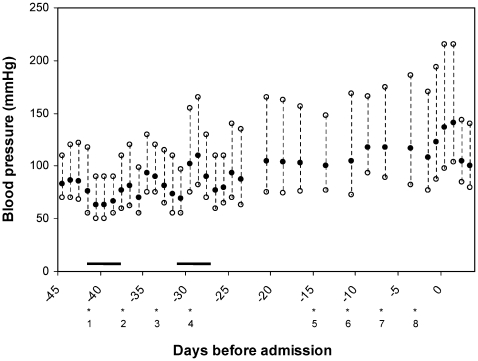
In November 2007 the multiple myeloma relapsed and was accompanied by renal failure due to cast nephropathy for which she required haemodialysis. She also had ascites containing myeloma cells. It was decided to treat the relapsed myeloma with bortezomib in combination with dexamethasone, which was started at the end of December 2007. Bortezomib (2 mg intravenously) was given twice weekly (at day 1, 4, 8 and 11) in a 21 day cycle. Dexamethasone (40 mg once daily for four consecutive days) was added on day 1–4 and 15–18. Three months after the initiation of bortezomib and 3 days after the eighth infusion during the second treatment cycle she presented at the emergency room with a severe right sided headache, which started about 3 h before presentation. Her family added that since the onset of the headache she had visual disturbances, was unable to speak coherently, and had periods of confusion. No seizures had been observed. In retrospect, it was observed that during the first cycle of bortezomib treatment her blood pressure remained in the normal range (mean 130/70 mm Hg). However, during the second cycle her blood pressure increased while her antihypertensive medication remained unaltered (fig 1). There were no significant differences between pre- and post-dialysis blood pressure levels during this period.
Figure 1.
Blood pressure during bortezomib treatment. Bortezomib infusions (2 mg intravenously, eight in total) are indicated with *, 1 = first infusion, 2 = second infusion, etc. The black bars indicate dexamethasone treatment, 40 mg once daily during 4 consecutive days.
Investigations
At presentation, physical examination revealed no abnormalities, except for a blood pressure of 183/94 mm Hg. Neurologic examination showed altered consciousness and Anton’s syndrome was positive (denial of visual inability associated with cortical blindness). Fundoscopy could not be carried out because of continuous blinking. There were no lateralising or focal abnormalities. Nuchal rigidity was absent.
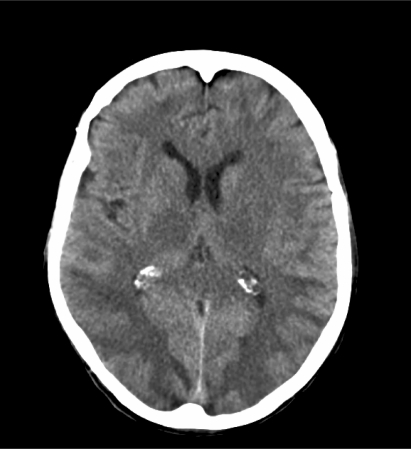
Laboratory evaluation was unremarkable except for a serum creatinine of 352 μmol/l (3.98 mg/dl) and a blood urea nitrogen of 12.3 mmol/l (34.4 mg/dl). A cerebral computed tomography (CT) scan showed hypodense areas in the subcortical and parieto-occipital white matter (fig 2), consistent with RPLS. There was no evidence of stroke or metastatic disease.
Figure 2.
Cerebral computed tomography scan at presentation showing bilateral hypodense areas in the subcortical and parieto-occipital white matter, characteristic of reversible posterior leucoencephalopathy syndrome.
Differential diagnosis
RPLS due to bortezomib
Hypertensive encephalopathy
Treatment
After admission, immediate treatment with intravenous labetolol was started with the aim to initially lower the mean arterial blood pressure in a controlled way by at least 25%. Treatment with bortezomib was withheld. Soon after admission the patient developed epileptic seizures that were successfully treated with lorazepam.
Outcome and follow-up
During hospital follow-up no further seizures occurred. As soon as her blood pressure normalised to values around 140 mm Hg systolic and 90 mm Hg diastolic, which was about 3 days after admission, the neurological symptoms disappeared. At this time she was alert and able to read and speak coherently with her family. Several days later, however, she became progressively dyspnoeic due to pleural effusion caused by progression of the myeloma. She died soon thereafter.
Discussion
RPLS, first recognised in 1996 by Hinchey et al,5 is an uncommon, potentially fatal clinico-radiological syndrome related to severe hypertension and the use of cytotoxic drugs. Symptoms include headache, altered mental functioning, visual changes and seizures in association with bilateral posterior cerebral white matter lesions.5 The white matter lesions associated with RPLS are a result of vasogenic oedema and disappear after blood pressure is lowered and, if present, the offending agent is removed.5,6 The typical posterior involvement of the brain has been associated with the relatively scarce sympathetic innervation of the outflow tract of the vertebral arteries and an increased vulnerability for the effects of cerebral hyperperfusion.7 Because of the close time relationship we believe that the occurrence of RPLS in this patient was attributable to bortezomib. Although hypertensive encephalopathy is an established cause of RPLS,6 we believe that the increase in blood pressure was not high enough to act as the single cause of RPLS. We propose that the combination of an elevated blood pressure together with the additional effects of bortezomib contributed to the development of RPLS.
Bortezomib, a 26S proteasome inhibitor, was approved by the US Food and Drug Administration (FDA) in 2003 for the treatment of relapsed multiple myeloma, based on the results from the SUMMIT phase 2 trial.8 Bortezomib blocks protein degradation by the proteasome and thus inhibits activation of the transcription factor nuclear factor-kappaB (NF-κB) leading to decreased transcription of cellular growth factors, proangiogenic factors such as vascular endothelial growth factor (VEGF), and anti-apoptotic factors. This ultimately leads to a direct cytotoxic effect and increased susceptibility of cancer cells, including multiple myeloma cells, to chemotherapeutic agents and a decrease of angiogenesis.9 Recently, promising results appeared from a phase 3 study showing beneficial effects for bortezomib added to conventional melphalan–prednisone treatment in patients with newly diagnosed myeloma, who were ineligible for high dose therapy.10 Reported side effects of bortezomib treatment include gastrointestinal complaints, transient thrombocytopenia, fatigue, fever (mostly sensoric), peripheral neuropathy and, less common, orthostatic hypotension and hypertension.9
Bortezomib has been previously associated with RPLS.11 In this case report, a 66-year-old patient presented with generalised tonic–clonic seizures and an altered mental status. He was moderately hypertensive while the neurologic examination was unremarkable. Magnetic resonance imaging (MRI) of the brain showed extensive asymmetrical high signal in the subcortical white matter of the occipital lobes, consistent with posterior leucoencephalopathy. After discontinuation of bortezomib and starting antihypertensive treatment, the symptoms resolved and an MRI performed 3 months later was normal. Interestingly, other antiangiogenic agents such as bevacizumab, a monoclonal anti-VEGF antibody approved for advanced colon, breast and non-small cell lung cancer, and sorafenib, a multi-targeted kinase inhibitor with VEGF inhibiting properties approved for renal cell and hepatocellular carcinoma, have also been associated with RPLS.1–4 The development of RPLS associated with these antiangiogenic agents typically follows prolonged administration of the drug varying between three and six cycles of therapy.
The reported associations between bortezomib and other VEGF inhibitors with the development of RPLS are in sharp contrast with the protective effects of VEGF inhibitors on the blood–brain barrier. VEGF, previously known as vascular permeability factor, downregulates endothelial transmembrane tight junction proteins.12 The increased vascular permeability facilitates the delivery of oxygen and nutrients to brain tissue, but also leads to the development of vasogenic oedema.7 Inhibition of VEGF limits oedema formation and may protect against cerebral oedema in the acute phase of stroke or cerebral venous infarction.13,14 It is conceivable that the acute protective effects on vascular permeability are negated with the prolonged use of anti-VEGF therapy. These long term negative effects on the blood–brain barrier associated with anti-VEGF therapy may include the development of hypertension and apoptosis of endothelial cells.
Anti-VEGF therapy has been shown to elicit hypertension probably by inhibition of endothelial mediated production of nitric oxide (NO), which leads to an increased vascular tone.15 Hypertension per se is an important risk factor for RPLS and can act as a single cause for its development.5 However, the blood pressure values observed in our and other patients with anti-VEGF therapy associated RPLS are usually lower than that observed in patients with RPLS due to hypertensive encephalopathy. Therefore we propose that apoptosis of endothelial cells and inhibition of VEGF-induced production of new endothelium may result in failure to maintain normal vascular tone or facilitate disruption of the blood–brain barrier, or both. As the apoptotic effects of anti-VEGF therapy on the endothelium are dose and time dependent it may explain why RPLS is only observed after prolonged administration of anti-VEGF therapy.9
Clinicians should be aware of the potential association between RPLS and newly designed targeted therapies with anti-angiogenic properties such as bortezomib. Timely recognition and appropriate treatment are important in the prevention of irreversible complications including severe neurological damage.
Learning points
Clinicians should be aware of the potential association between RPLS and newly designed targeted therapies with anti-angiogenic or neurotoxic properties such as bortezomib and bevacizumab.
Apart from measuring blood pressures alone, a more intensive monitoring may be required if patients are treated with these agents, in order to prevent dramatic complications such as RPLS.
Prompt recognition of RPLS followed by immediate (intravenous) blood pressure lowering treatment and discontinuation of anti-VEGF therapy may result in rapid clinical improvement.
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication
REFERENCES
- 1.Glusker P, Recht L, Lane B. Reversible posterior leucecephalopathy syndrome and bevacizumab. N Engl J Med 2006; 354: 980–1 [DOI] [PubMed] [Google Scholar]
- 2.Koopman M, Muller EW, Punt CJA. Reversible posterior leucencephalopathy syndrome caused by bevacizumab: report of a case. Dis Colon Rectum 2008; 51: 1425–6 [DOI] [PubMed] [Google Scholar]
- 3.Peter S, Hausmann N, Schuster A, et al. Reversible posterior leukoencephalopathy syndrome and intravenous bevacizumab. Clin Experiment Opthalmol 2008; 36: 94–6 [DOI] [PubMed] [Google Scholar]
- 4.Govindarajan R, Adusumilli J, Baxter DL, et al. Reversible poserior leucoencephalopathy syndrome induced by RAF kinase inhibitor BAY 43–9006. J Clin Oncol 2006; 25: e48. [DOI] [PubMed] [Google Scholar]
- 5.Hinchey JA, Chaves C, Appignani B, et al. A reversible posterior leucencephalopathy syndrome. N Engl J Med 1996; 334: 494–500 [DOI] [PubMed] [Google Scholar]
- 6.Schwartz RB, Jones KM, Kalina P, et al. Hypertensive encephalopathy: findings on CT, MR imaging and SPECT imaging in 14 cases. AJR Am J Roentgenol 1992; 159: 379–83 [DOI] [PubMed] [Google Scholar]
- 7.Edvinsson L, Owman C, Sjöberg NO. Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res 1976; 115: 377–93 [DOI] [PubMed] [Google Scholar]
- 8.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003; 348: 2609–17 [DOI] [PubMed] [Google Scholar]
- 9.Roccaro AM, Hideshima T, Raje N, et al. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial cells. Cancer Res 2006; 66: 184–91 [DOI] [PubMed] [Google Scholar]
- 10.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008; 359: 906–17 [DOI] [PubMed] [Google Scholar]
- 11.Kelly K, Kalachand R, Murphy P. Bortezomib-induced reversible posterior leucoencephalopathy syndrome. Br J Haematol 2008; 141: 566. [DOI] [PubMed] [Google Scholar]
- 12.Argaw AT, Gurfein BT, Zhang Y, et al. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci USA 2009; 106: 1977–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi OZ, Hunter C, Liu X, et al. Effects of VEGF and nitric oxide synthase inhibition on blood-brain barrier disruption in the ischemic and non-ischemic cerebral cortex. Neurol Res 2005; 27: 864–8 [DOI] [PubMed] [Google Scholar]
- 14.Kimura R, Nakase H, Tamaki R, et al. Vascular endothelial growth factor antagonist reduces brain edema formation and venous infarction. Stroke 2005; 36: 1259–63 [DOI] [PubMed] [Google Scholar]
- 15.Sane DC, Anton L, Brosnihan KB. Angiogenic growth factors and hypertension. Angiogenesis 2004; 7: 193–201 [DOI] [PubMed] [Google Scholar]