Abstract
Little is known of the life-history of many parasitic species. This hinders a full understanding of host-parasitic interactions. The common swift louse fly, Crataerina pallida Latreille (Diptera: Hippoboscidae), an obligate haematophagous parasite of the Common Swift, Apus apus Linnaeus 1758, is one such species. No detrimental effect of its parasitism upon the host has been found. This may be because too little is known about C. pallida ecology, and therefore detrimental effects are also unknown. This is a review of what is known about the life-history of this parasite, with the aim of promoting understanding of its ecology. New, previously unreported observations about C. pallida made from personal observations at a nesting swift colony are described. Unanswered questions are highlighted, which may aid understanding of this host-parasite system. C. pallida may prove a suitable model species for the study of other host-parasite relationships.
Keywords: Apus apus, avian parasites
Introduction
In order to understand host-parasite systems, the life-history of the parasite species being studied needs to be well known. However, for many parasitic species information about basic biological traits is missing. This lack of knowledge could be hindering a full understanding of host-parasite relationships. Although a number of studies have shown that parasites do have an effect on their hosts (reviewed: Møøller et al. 1990; Lehmann 1990; Møøller 1997) other studies have shown no such effect (e.g. Johnson and Albrecht 1993; Clayton and Tompkins 1995; Lee and Clayton 1995; Eeva et al. 1994). This apparent lack of pathogenicity may be because of a lack of knowledge of parasite life-history.
The common swift louse fly Crataerina pallida Latreille (Diptera: Hippoboscidae) may be an excellent example of a parasitic species where no apparent pathogenetic effect has been found, but this may be because of such a lack of detailed knowledge of its life-history. This is an obligate avian nest ectoparasite of the common swift Apus apus Linnaeus 1758. However, despite being relatively large, tractable, and having a host species that is common and widely distributed throughout Europe, surprisingly little is known of their biology (Marshall 1981). Much of what is known is scattered among the scientific literature is of substantial age or is in a language other than English, the current hegemonic language of science. Studies have failed to find an effect of its parasitism upon its host (Lee and Clayton 1995; Tompkins et al. 1996).
This is the first review of what is known about this parasite species. This review aims to collate life-history information about C. pallida and highlight questions requiring further study in order to promote a better understanding of this host-parasite system. New observations made from personal experiences with C. pallida from a nesting colony of the common swift situated beneath a roadway bridge close to the town of Olpe, Germany (51°° 04′? 00″? N, 07°° 81′? 00″? E) (Site described by Walker et al. 2009) are described. Several features not previously observed are described.
C. pallida may prove to be an excellent model species of a nest ectoparasite, and many of the themes and problems raised may also apply to other host-parasite systems. There are many possible advantages of C. pallida as a model nest parasite species, including its large size and easy tractibility, which make conducting experimental work and quantifying levels of parasitism relatively easy compared with other types of nest parasite. It is hoped that this review will prompt investigations of the life-history traits of other host-parasite systems.
Taxonomy
Louse flies belong to the Hippoboscidae family of cyclorrhaphous insects within the Suborder Brachcera. Hippoboscids are viviparid haematophagous obligate ectoparasites of mammals and birds (Hutson 1984). Formerly the Hippoboscidae were classified along with the bat fly families Nycteribiidae and Streblidae within the single grouping of the Pupipara.
The Hippoboscidae family contains 213 species, and is divided into three subfamilies with 21 genera (Hutson 1984). This family contains a number of well-known and common parasitic species of birds and mammals; for example the avian louse fly Ornithomya avicularia from the Ornithomyinae subfamily is a common parasite of a variety of bird species. The Hippoboscinae subfamily contains the horse ked Hippobosca equine. The Lipopteninae subfamily contains the deer ked Lipoptena cervi and the sheep ked Melophagus ovinus. Those species of Hippoboscids that parasitize birds are commonly known as ‘‘louse flies,’’ while those that parasitize mammals, although similar to their avian counterparts, are known as ‘‘keds’’ (Hutson 1984). Most Hippoboscid species occur in the Old World tropics, but 16 species occur in Europe, seven of these on avian hosts (Hutson 1984).
There are eight species within the genus Crataerina, three of which occur in Europe. C. pallida parasitizes the common swift A. apus and the pallid swift A. pallidus; C. melba parasitizes the alpine swift A. melba; and C. hirundinis parasitizes the house martin Delichon urbicum.
Physical characteristics of C. pallida
This species possesses a number of features that aid attachment to its host and reduce the chance of removal through host grooming. It has the standard Arthropod bauplan with there being three tagma —— a distinct head, thorax, and abdomen. The entire body is dorsoventally flattened, which allows it to burrow with ease right to the base of bird feathers and reach its source of food. The exoskeleton is tough, protecting them from being crushed by the host.
The thorax and abdomen are covered with short sharp black hairs, which are also found on the legs and head capsule, and these presumably get caught on the barbs of feathers and provide points of attachment to the host. They are particularly prominent on the posterior abdomen. The joints between the legs are shaped like short sharp hooks, and the legs themselves end in three sharp claws that are ideal for attachment. Adult C. pallida have no difficulty in walking upside down across glass or plastic surfaces. The head is sunk into the thorax, and the mouthparts are partially retractable, which protects them from abrasion with the host integument (Lehane 1991).
As for many Hippoboscid flies, C. pallida has atrophied vestigial wings that are borne on the thorax and are not capable of sustaining powered flight. A number of Hippoboscid species do retain functional wings, for example the horse ked H. equine. Some species lose their wings on finding a host, such as Allobosca spp. where the wing tips are lost or the deer fly L. cervi where the wings are lost entirely once a suitable host is found (Lehane 1991). C. pallida is closely associated with their hosts' nests, and therefore an ability to fly is probably not necessary. The wings have probably not degenerated completely because of their value in providing another type of ‘‘hook’’ to allow attachment to the host.
The head capsule of the Hippoboscidae has become specially adapted for its haemophagous diet, but is nevertheless similar in structure to that seen in the Muscidae (Bequaert 1953). The mouthparts form a distinct prognathous which is found on the ventral midline of the head capsule and ends in a closed sclerotized tube or torma. As in all cyclorrhaphids, there is a cibarial pump. There is a pair of sensory antennae.
C. pallida are large insects, with females being larger than males. Fifteen female and 14 male engorged adult louse flies were measured during July 2008. The 15 engorged females had a body length of 7.43 mm (SD ±± 0.455), average abdomen width of 5.45 mm (SD ±± 0.53), and abdomen length of 4.01 mm (SD ±± 0.36). Males were smaller with an average body length of 7.16 mm (SD ±± 0.49), abdomen width of 3.78 mm (SD ±± 0.41), and abdomen length of 4.58 mm (SD ±± 0.42). This difference in size is not simply due to the fact that females can store a larger volume of blood. Females have been found to be larger than males both in the engorged and unengorged states (Kemper 1951). Females probably have to be larger than males as they are the sex which produces eggs and provision the larvae internally.
The legs are held away from the body when at rest, and this gives C. pallida a characteristic ‘‘spider’’ or ‘‘star-like’’ stance. In colouration, the adult imagines are a light to dark brown colour. Teneral specimens have a translucent sheen, which is, however, soon lost. In imagines that have fed, the abdomen is noticeably larger and more swollen and is a light to dark grey colour. C. pallida with dark red coloured abdomens are occasionally seen, and these have presumably recently fed.
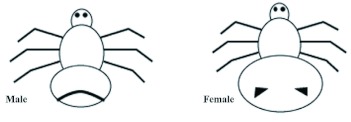
Differentiating between the sexes of engorged C. pallida is easily done with the naked eye (Kemper 1951). In males, a black, semi-circular ring is present on the rear of the abdomen. Females instead have two spot-like triangular black marks. Females have much larger, wider, more engorged abdomens than the males. Males are hairier than females. Discriminating between males and females that have not fed is more difficult. Males have more heavily segmented abdomens than the females, but a magnifying scope is needed to see this. The genitalia of male C. pallida can be exposed by gently pressing on the abdomens of the males and thus facilitating sexing.
Figure 1.
Schematic diagram showing differences between Crataerina pallida sexes after Kemper (1951). High quality figures are available online.
Lifecycle
There is a strong association between the lifecycle of C. pallida and that of the host's breeding season. 4th instar imagines emerge synchronously with the return of the common swift in spring. Pupae are cyclorrhaphous. Although emergence has been found to coincide with the hatching of swift nestlings (Büütiker 1944; Lack 1956), others have found that it occurred earlier (Hutson 1981; Bromhall 1980). In 2007, the first C. pallida emerged on 15 May, during the period of swift egg laying (e.g. Figures 2 and 3). In 2008, C. pallida had emerged before the 03 June, when nestlings began to hatch. Weather conditions may influence the exact timing of emergence of C. pallida.
Figure 2.
Adult Crataerina pallida at the nest during the incubation period of the Apus apus eggs. High quality figures are available online.
Figure 3.
A nest particularly heavily parasitized by adult Crataerina pallida. There are approximately 20 adult C. pallida in this nest. High quality figures are available online.
The emergence from the pupae appears to be temperature mediated. Anecdotal reports suggest that pupae left on a radiator began to hatch after several days (Kemper 1951). In a more analytical study, emergence of the house martin louse flies occurred more rapidly at elevated temperatures (Popov 1965).
Sixty pupae were collected from a C. pallida colony in 2008 and divided into three groups of 20 pupae, and from 03 October, each group was kept either continuously at room temperature at approximately 20°°C, within a refrigerator (mean temperature 5°°C), and within one of two warm cabinets (mean temperatures of 24 and 47°°C).
Adults emerged from 9 of the 20 pupae kept at room temperature between 27 April and 02 May, somewhat earlier than what would be expected. The group kept in the refrigerator hatched between August and October the following year, much later than would occur under normal conditions. Emergence occurred at none of the pupae kept in the warmest warm cabinet, probably because the temperatures experienced were lethal. However, emergence of those kept in the cooler warm cabinet occurred between the 27 and 30 April with C. pallida emerging from 7 of 20 pupae. Six of 20 pupae kept in a refrigerator for 3 months from August to November in order to simulate ‘‘winter,’’ and thereafter at room temperature to simulate ‘‘spring’’, began to hatch in mid-February, roughly three months earlier than normal, thus indicating that a period of winter cooling may be necessary for emergence to occur.
Mating of C. pallida usually takes place on or in close proximity to the nest, but may also occur on the adult or nestling swifts. As in bat flies (Strebilidae and Nycteribiidae), blood ingestation may be necessary for succesful copulation to occur (Yuval 2006). Mate guarding seems to occur, with male C. pallida sometimes remaining mounted on the females for several minutes at a time. Two or three C. pallida males may attempt to mount a single female. Mating competition may increase as the summer progresses perhaps due to the limited amount of time available before swift departure and due to the falling number of females. ‘‘Clusters’’ of C. pallida often occur in which more than 20 C. pallida may congregate together in one large mass (Figure 3).
Female M. ovinus are able to store enough sperm after a single mating to fertilise all their subsequent eggs (Evans 1950; Small 2005). Should this prove to be the case with Crataerina species, it might mean that males able fertilise females first could be at a significant advantage than later emerging males. This may explain why males hatch from the winter diapause earlier than the females. It may also help explain the female dominated sex ratios seen during the summer, as there may be no advantage for males in staying alive after they have copulated. Their presence may increase the parasitic burden on the hosts that their own offspring will ultimately rely on.
Larvae develop singly within the female's uterus in a mechanism known as adenotrophic viviparity. Larvae are nourished through special milk glands found within the common oviduct (Baker 1967) and, if development is similar to that of other Hippoboscid species, takes approximately 3 weeks (Small 2005). Larvae are deposited when they reach the third instar, and they then pupate almost immediately (Baker 1967). Larvae are deposited either underneath or some distance away from the nest (Figure 4). In comparison, other Hippoboscidas deposit pupae at no specific location, for example those of the genus Lipoptera, or the pupae are purposely attached to the host as is the case in M. ovinus (Lehane 1991). On deposition, pupae are a light brown colour and require six hours to become hardened and dark in colouration.
Figure 4.
Pupae deposited to the side and beneath the nest. Two of the pupae are a dark brown in colouration, indicating that they have only recently been deposited. Pupae are typically black in colouration. Beneath the nest to the right is a small aggregation of adult Crataerina pallida that may be the result of mating competition. High quality figures are available online.
Hippoboscids have relatively low fecundity. It is unknown how many larvae a single female can produce, but female sheep keds can produce new larva every 6 to 8 days, and so can therefore probably produce between 12 and 15 larvae over the course of a lifetime (Small 2005). A similar figure in Crataerinids is likely. Other Hippoboscids have lifespans of between 6 and 10 weeks (Lehane 1991; Small 2005). The number of pupae seen at the nest has been found to be higher at the end of July than in June (Kemper 1951). This indicates that most pupal production occurs during the month of July, during the nestling period. Pupae remain in diapause until the following spring.
Basic life-history information about C. pallida is missing, for example information on the lifespan of adults, the number of pupae females are capable of producing, and the factors affecting adult emergence each spring.
Population dynamics
Population size. At the study site, the population of C. pallida found at the nests during 2007 peaked during mid-May, which coincided with the incubation of the eggs. In 2008, C. pallida numbers peaked during the incubation and were falling by the time the nests could be first examined at the end of incubation. Throughout the nestling period of both years, the number of C. pallida seen steadily dropped. A similar pattern has been reported for C. hirundinis (Bequaert 1953). Studies on the number of C. pallida on captured adult birds also show a decrease in numbers as the summer progresses (Hutson 1981).
A. apus pairs are nest-site faithful, often returning year after year to the same nest site (Weitnauer 1947; Lack 1956). This may affect C. pallida populations, allowing them to increase on a year by year basis at individual nests with progressive use. At the study site, new and young nests do appear to be less heavily parasitized than more obviously older, well-established nests, although not enough time has passed to show this conclusively. It may be the case that a build-up of parasite numbers over several years may be a factor causing nest abandonment and the establishment of new nests in an attempt to forego parasitism.
Other factors, such as the weather or climate, may also influence C. pallida numbers. A correlation between the abundances of a louse fly species on Serins, Serinus serinus, and the weather has been seen (Summers 1975). Recently fledged nestlings of the north island robin Petroica australis were more likely to be parasitized by C. pallida if they came from wetter territories (Berggren 2005).
The number of C. pallida seen at particular nests can vary considerably on a day by day basis. This may be due to C. pallida moving onto and off the adult hosts and thus being removed temporarily from the nests. This, along with the general changes in C. pallida numbers that occur throughout the swift breeding season may lead to a false picture of the true intensity of parasitism being made if the population is sampled on only a small number of occasions. Data on the consistency of C. pallida populations over the entire season and on a day-by-day basis is needed.
Another factor which may influence the population size of C. pallida seen at a nesting colony is the size of the colony involved. Generally speaking larger nesting aggregations of birds are more heavily parasitized. Whether this occurs with Crataerina spp. is difficult to decipher, as relatively few colonies have been studied. The population of C. pallida seen at the well studied Oxford colony of the common swift is smaller in size than that seen at the study site despite the fact that it houses considerably more nesting swifts.
Host predation may be a major cause of Hippoboscid mortality (Hutson 1984). However, this is not the case for C. pallida. Adult A. apus are reported to ignore adult louse flies and to take no measures to remove them from themselves (Lack 1956; Bromhall 1980). A. apus nestlings do not feed on adult C. pallida. Should an A. apus manage to preen a C. pallida with its beak, the parasite will simply wait until the bird opens its mouth and crawl out (G. Candelin personal observation). Ironically, C. pallida may be the prey of a parasitic wasp. Two species of Hymenoptera of the Pteromalidae family, Nasonia vitripennis and Dibrachys cavus have been reared from the puparia of C. pallida and maybe also C. hirundinis (Bequaert 1953).
Aggregation and prevalence. Parasitic species typically exhibit aggregated population distributions. This is the case for C. pallida (Hutson 1981) and for C. melba (Tella & Jovani 2000), although the level of aggregation seen by these species is lower than seen in other host-parasite systems.
The prevalence of parasitism exhibited by louse fly species is much higher than is normally seen in other parasites. On adult alpine swifts infestation rates by C. melba of 70.8% (Tella and Jovani 2000) and of 74% (Tella et al. 1995) averaged over the summer were found. On A. apus adults parasitized by C. pallida the average infestation over the entire season was 34.4% (Hutson 1981), and at A. apus nests 67% (Tompkins et al. 1996). For comparison, the prevalence of the louse fly Ornithomyia avicularia, on serins S. serinus, was found to be 3% (Senar et al. 1994), and the prevalence of other Hippoboscid flies on other species has been shown to be no greater than 20% (McClure 1984).
The infestation rate of adult swifts has been found to vary with date, being at around 10% in early spring, raising quickly to 50% during the incubation period, and reaching a maximum of 50% to 60% around the time of nestling hatching, before declining rapidly during the second period of nestling growth (Hutson 1981). These changes can probably be explained through changes to the A. apus lifecycle, with infestation being highest during incubation when A. apus are at the nest for the longest periods, and falling when they are feeding the young and are there less often. It has been proposed that the high prevalence of louse flies on swifts could be due to their short legs and lack of easily moveable head, which prevents birds from effectively removing parasites (Tella et al. 1998).
The prevalence of C. pallida and their intensity of parasitism has been determined at only one nest site, at the Oxford University Museum site used in the original study by Lack (1956). At this study site, a mean parasitic intensity of only one adult C. pallida per nest has been found, with the maximum number in any one nest being 9 adult C. pallida (Lee and Clayton 1995). At the study colony, where nests are left in place between breeding seasons, the maximum number of C. pallida seen in a single nest in 2007 was 27, and the average number of C. pallida seen per nest was 3.64 (SD ±± 2.65). These figures are substantially higher than those seen at Oxford. However, it is usual at the nesting site at the museum for nests to be removed on a yearly basis (G. Candelin personal communication). This may lead to a distortion of louse fly populations and to an artificially lower number of parasites per nest than would normally occur. It has been shown that the removal of old, heavily parasitized nests affects the distribution and intensity of parasitism in nest box studies (Møøller 1989). The removal of nests and the resulting unnaturally lower levels of parasite abundance seen may be the reason why studies at Oxford failed to find any negative costs of C. pallida parasitism.
Sex ratio. Louse fly populations are female-biased. More female than male C. hirundinus were found at house martin nests and on adults (Summers 1975; Popov 1965; Hardenberg 1929); likewise for C. melba at alpine swift nests (Tella and Jovani 2000). A greater proportion of female than male C. pallida has been seen on adult A. apus (Hutson 1981). This female bias is puzzling as an equal number of males and females are thought to hatch (Bequaert 1953). Other Hippoboscids, such as M. ovinus, have more equal sex ratios (Small 2005). Distinct differences in the sex ratio at different stages of the summer have been found (Kemper 1951). In spring, female C. pallida were seldom found on adult A. apus. The proportion of males found dropped rapidly as incubation began. This may be due to males emerging and then dying off before females (Kemper 1951). This idea tallies with observations of pupae in the lab, where males consistently emerged first.
Tella and Jovani (2000) found that the ratio of male and female C. melba louse flies on hosts was inter-connected with mate attraction being one possible cause. As mating competition appears to be strong in C. pallida, this may also be a factor influencing sex ratios and population dynamics. The effect of such mate attraction as a factor affecting parasite population biology, and thus pathogenicity, has rarely been looked at, and this species may therefore prove an ideal model species for such studies.
Transmission and dispersal. When adult A. apus return from overwintering sites in Africa, they are C. pallida-free (Zumpt 1966). Therefore, an easy way for A. apus to avoid C. pallida parasitism would be to build a new nest in a C. pallida-free place. Where C. pallida have been marked, it has been seen that although C. pallida could move between nests, this rarely occurred, with only 6 from 96 flies moving to adjacent nests (Summers 1975). Whether this was active dispersal by C. pallida themselves or whether they were carried between nests could not be determined. C. pallida have no mechanism themselves to move between nests discretely separated from each other or to new colonies some distance away from existing ones. Transmission has been assumed to be vertical based on these results (Lee and Clayton 1995; Tompkins et al. 1996). However, this study showed only that C. pallida are unlikely to move to other nests under their own locomotion and did not preclude them being carried to other nests by nestlings or adult A. apus.
During the breeding season when the nestlings are at the nest, transmission is undoubtedly vertical. However, once the nestlings fledge, they can be no longer be re-infected with C. pallida from the natal nest, and when they return from the winter migration, they are C. pallida-free. Thereafter, transmission of C. pallida must be horizontal and occur from adult to adult, or from adult to nest to adult. Most likely is that C. pallida are transmitted to new sites through adult A. apus or first year adults that visit new or existing nest sites and carry C. pallida with them. A greater proportion of female than male C. pallida were found on adult house martins (Summers 1975), which may be the result of females feeding more often than males, but could also be because gravid females actively transfer onto adults as doing so they may be dispersed to new sites where they can deposit their pupae. Females acting in such a way as to facilitate their own dispersal would increase their lifetime reproductive success if they managed to get transferred to a new formerly uncolonised nest site which they and their offspring could successfully inhabit without experiencing intra-specific competition.
Parasitism
Pathogenicity. No pathogenic effect of C. pallida parasitism on their A. apus hosts has been found (Lee and Clayton 1995; Tompkins et al. 1996; Hutson 1981). This is surprising. C. pallida feed once every 5 days, males taking 23 mg, and females 38 mg of blood (Kemper 1951). It has been calculated that if the total blood volume is estimated as being 10% of total body weight; then in an adult A. apus weighing 42 grams, this represents about 5% of its blood being lost (Campbell 1988). Therefore, substantial quantities of blood may be lost.
Adult A. apus with heavy infestations had weights within the normal weight range of adult swifts leading to one author to conclude that there was no evidence that heavy C. pallida infestation affected adult condition (Hutson 1981). There are anecdotal reports of grounded A. apus having C. pallida (Büütiker 1944; Lack 1956); however, this is hardly strong evidence for a negative effect of these parasites. No correlation between C. pallida intensity and nestling body mass, the fledgling date, or the number of chicks fledged from each nest has been found (Lee and Clayton 1995). Where C. pallida abundances were artificially manipulated, no differences in nestling growth or fledging success was seen (Tompkins et al. 1996). Although no pathogenic effect has been found on A. apus, a number of studies have found an adverse effect of the closely related louse fly, C. melba, on the Alpine Swift (Bize et al. 2003; Bize et al. 2004; Bize 2005).
The type and level of transmission and transfer of parasites between hosts is important in influencing the level of parasite virulence seen (Bull 1994). Parasites that transfer between hosts in a mainly vertical manner, from parent to offspring, typically exhibit lower levels of pathogenicity than parasites that transfer between unrelated hosts horizontally (Eward 1994). Tompkins et al. (1996) postulated that the lack of virulence seen by C. pallida may be due to the vertical nature of its transmission. However, horizontal transmission also occurs and is commonly reported at colonies where nests are situated close together (e.g. Bize et al. 2003; Bize et al. 2005). C. pallida which have not fed have been shown to be more active than those that have (Miller 1997), and thus may be more likely to transfer between closely situated nests where these are available. The pathogenicity of C. pallida may be dependant and may alter depending on the nature of the nest colony at which it is found; because of this C. pallida may prove an interesting model species for looking at the evolution and development of parasite transmission and pathogenicity.
By looking for more subtle effects of parasitism, such as compensatory growth during the nestling phase, the sex ratio of fledging nestlings, and the lifespan and reproductive success of adult parent birds effects of parasitism by C. melba on the Alpine Swift have been found (Bize et al. 2003; Bize et al. 2004; Bize et al. 2005). Saino et al. (1998) found that the speed of growth of Barn Swallow nestling wings was influenced through parasitism by the O. biloba louse fly. Future studies investigating C. pallida parasitism should likewise look at such finer aspects of A. apus reproductive success and not simply on the more obvious parameters such as adult weight, nestling fledging weight, and nestling survival, as has been before. More direct effects of parasitism, such as parasite caused anaemia, have yet to be reported but are likely to occur as a result of the blood loss experienced by hosts parasitized by C. pallida.
Mode of parasitism. Crataerinid louse flies, unlike other types of louse flies such as O. avicularia, are monoexous, being host specific (Kemper 1951; Tella and Jovani 2000). However, in addition to parasitizing A. apus, C. pallida is also reported to parasitize the pallid swift (M. Cucco personal communication). The development of host specificity within louse fly-avian parasite systems may be worth investigating further. Is there any separation in the Crataerina populations parasitizing Common and Pallid Swifts? Could divergence occur in the future?
When initiating feeding, C. pallida dive between the feathers to reach the skin. Feeding C. pallida appear somewhat like ticks, with the heads being burrowed into the host, while the legs and abdomen protrude outwards. When they finish feeding, they move backwards away from the skin of the host, before delving into a new position to feed. On nestlings, they are often found feeding on the lower rump area. On adults, they are reported to feed preferentially on the belly and neck (Kemper 1951). C. pallida which have not fed have abdomens that are noticeably smaller and have a light brown colouration. In adults that have fed, the abdomen is substantially larger and has a greyish colouration.
Host selection. When faced with a brood of chicks parasites have to choose one, and they may be different. Although large nestlings may offer large resources, they will have strong immune responses; weak nestlings on the other hand will offer fewer resources but will be less able to invest in immune defences (reviewed: Sheldon and Verhulst 1996). Louse flies are an ideal parasite to study these trade-offs. Host preference of C. melba has been found to be linked to nestling age, preferring older siblings with more developed feathers (Roulin 2003). Later when there was little difference in feather development between nestlings, these preferences disappeared and no nestling was favoured. Conversely, a later study found that nestlings intermediate in size were preferred, perhaps a compromise choice between nestling resources and immune response (Bize et al. 2008).
Typically host-parasite studies consider the effects of parasitism on the level of the individual. However, in the case of C. pallida, and maybe other nest parasites, a more appropriate level of study may be to consider each nest, with its associated parent and nestling birds, as being a discrete unit of parasitism. Attempts should also be made to try to explain features of parasite life-history in relation to their hosts and their hosts' life-histories. A parasite's life-history features may be tuned to those of its host, thus enhancing parasite fitness. To what extent are the skewed sex ratios, the declining population sizes, and the intense mating competition exhibited by C. pallida the result of C. pallida attempting to maximise their fitness in the face of the biology and breeding biology of their avian hosts? Future studies should consider aspects of parasite life-history as being adaptations to the host species on which they prey.
Vectors. It is known that Hippobiscid flies act as vectors of various species of Trypanosoma and Haemoproteus (Baker 1967; Bize et al. 2005). Crataerina spp. may also act as vectors of such parasites and such a role has been discussed (Soulsby 1968). C. pallida may engage in a phoretic association with feather mites (Astigmata), and thus aid their transmission (Jovani et al. 2001). Small numbers of feather mites have been found on louse flies collected from avian hosts (Hill et al. 1967). However, studies testing whether this could be the case have found no evidence that such ‘‘hitch hiking’’ occurs (Philips and Fain 1991).
Summary
The common swift louse fly, C. pallida, is a fascinating example of an avian nest parasite, with many puzzling life-history features. When trying to understand parasite life-cycles and ecology it is important to consider what is occurring to the host species and how this may be affecting the parasite, or in what way the parasite may be using the hosts own ecology to its own advantage. Considering the C. pallida from this perspective may lead to a better understanding of the strategies it uses.
The common swift louse fly C. pallida may prove to be an excellent model species for studying host-parasite systems. It offers a number of advantages to the parasite researcher including large size and the ease at which it can be manipulated. In comparison with other nest and avian parasites, its populations can be easily quantified and determined. C. pallida may also prove an excellent example of how hosts and parasites co-adapt, with the life cycle of C. pallida appearing to be well in tune with that of their hosts. Connecting parasite life-cycles to that of their hosts may lead to a better understanding of a wide range of host-parasite systems.
Acknowledgements
Thanks to George Candelin of the Oxford University Swift Research Project and to Marco Cucco of the University of Piedmont commenting on early drafts of this manuscript, and to two anonymous referees for their comments on the submitted manuscript.
References
- Baker JR. A review of the role played by the Hippoboscidae (Diptera) as vectors of endoparasites. The Journal of Parasitology. 1967;53(2):412–418. [PubMed] [Google Scholar]
- Bequaert JC. The Hippoboscidae or Louse flies (Diptera) of mammals and birds. Part I. Structure, physiology and natural history. Entomologica Americana. 1953;32:1–209. [Google Scholar]
- Berggren A. Prevalence and impact of the native blood-sucking louse fly (Ornithoica sp.) on the North Island Robin (Petroica australis longipes). Notornis. 2005;52:114–116. [Google Scholar]
- Bize P, Roulin A, Bersier L-P, Pfluger D, Richner H. Parasitism and developmental plasticity in Alpine swift nestlings. Journal of Animal Ecology. 2003;72(4):633–639. doi: 10.1046/j.1365-2656.2003.00734.x. [DOI] [PubMed] [Google Scholar]
- Bize P, Roulin A, Bersier L-F, Richner H. Additive effects of ectoparasites over the reproductive attempts in the long-lived alpine swifts. Journal of Animal Ecology. 2004;73(6):1080–1088. [Google Scholar]
- Bize P, Roulin A, Tella JL, Richner H. Female-biased mortality in experimentally parasitized Alpine Swift Apus melba nestlings. Functional Ecology. 2005;19(3):405–413. [Google Scholar]
- Bize P, Jeanneret C, Klopfenstein A, Roulin A. What makes a host profitable? parasites balance host nutritive resources against immunity. American Naturalist. 2008;171(1):107–118. doi: 10.1086/523943. [DOI] [PubMed] [Google Scholar]
- Bouslama Z, Chabi Y, Lambrechts MM. Chicks resist high parasite intensities in an Algerian population of blue tits. Ecoscience. 2001;8(3):320–324. [Google Scholar]
- Bromhall D. Devil birds. The life of the swift. Hutchinson: 1980. [Google Scholar]
- Bull JJ. Perspective; Virulence. Evolution. 1994;48:1423–1437. doi: 10.1111/j.1558-5646.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Büütiker W. Die Parasiten und Nestgååste des Mauerseglers (Micropus apus L.). Der Ornithologische Beobachter. 1944;41:25–35. [Google Scholar]
- Campbell TW. Avian Hematology and Cytology. Iowa State University Press; 1988. [Google Scholar]
- Clayton DH, Tompkins DM. Comparative effects of mites and lice on the reproductive success of rock doves (Columba livia). Parasitology. 1995;110(2):195–206. doi: 10.1017/s0031182000063964. [DOI] [PubMed] [Google Scholar]
- Eeva T, Lehikoinen E, Nurmis J. Effects of ectoparasites on breeding success of great tits (Parus major) and pied flycatchers (Ficedula hypoleuca) in an air pollution gradient. Canadian Journal of Zoology. 1994;72(4):624–635. [Google Scholar]
- Evans GO. Studies on the bionomics of the Sheep Ked, Melophagus ovinus L., in west Wales. Bulletin of Entomological Research. 1950;40:459–478. [Google Scholar]
- Ewald PW. Evolution of infectious diseases. Oxford University Press; 1994. [Google Scholar]
- Hardenberg JD. Beitrage zur Kenntnis der Pupiparen. Zoologisches Jahrbuch (Anatomie) 1929;50:497–570. [Google Scholar]
- Hill DS, Wilson S, Corbet GB. Mites associated with British species of Ornithomya (Diptera-Hippoboscidae). Journal of Medical Entomology. 1967;4(4):102–122. doi: 10.1093/jmedent/4.2.102. [DOI] [PubMed] [Google Scholar]
- Hutson AM, Burton JA, Stephens SGD. Some preliminary results of swift ringing at Beddington Sewage Farm, Surrey. London Bird Report. 1971;35:81–87. [Google Scholar]
- Hutson AM. The population of the louse fly, Crataerina pallida (Diptera, Hippoboscidae) on the European Swift, Apus apus (Aves, Apodidae). Journal of Zoology, London. 1981;194(7):305–316. [Google Scholar]
- Hutson AM. Keds, flat-flies & bat-flies (Hippoboscidae & Nycteribiidae). 1984. Handbooks for the identification of the British Insects. Volume 10 Part 7. Royal Entomological Society.
- Johnson LS, Albrecht DJ. Effects of haematophagous ectoparasites on nestling house wrens, Troglodytes aedon: who pays the cost of parasitism? Oikos. 1993;66(2):255–262. [Google Scholar]
- Jovani R, Tella JL, Sol D, Ventura D. Are Hippoboscid Flies a Major Mode of Transmission of Feather Mites? The Journal of Parasitology. 2001;87(5):1187–1189. doi: 10.1645/0022-3395(2001)087[1187:AHFAMM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kemper H. Beobachtungen an Crataerina pallida Latr. und Melophagus ovinus L. (Diptera, Pupipara). Zeitschrift fiir Hygiene (Zoologie) 1951;39:225–259. [Google Scholar]
- Kierans JE. A review of the phoretic relationship between Mallophaga (Phthiraptera: Insecta) and Hippoboscidae (Diptera: Insecta). Journal of Medical Entomology. 1975;12(1):71–76. doi: 10.1093/jmedent/12.1.71. [DOI] [PubMed] [Google Scholar]
- Lack D. Swifts in a tower. Methuen publishing; 1956. [Google Scholar]
- Lee PLM, Clayton DH. Population biology of swift (Apus apus) ectoparasites in relation to host reproductive success. Ecological Entomology. 1995;20(6):43–50. [Google Scholar]
- Lehane MJ. Biology of Blood-sucking Insects. Cambridge University Press; 1991. [Google Scholar]
- Lehmann T. Ectoparasites: direct impact on host fitness. Parasitology Today. 1993;9(1):8–13. doi: 10.1016/0169-4758(93)90153-7. [DOI] [PubMed] [Google Scholar]
- Marshall AG. The ecology of ectoparasitic insects. Academic press; 1981. [Google Scholar]
- McClure HE. The occurance of Hippoboscid flies on some species of birds in southern California. Journal of Field Ornithology. 1984;55(2):230–240. [Google Scholar]
- Møøller AP. Parasites, predators, and nest boxes: facts and artefacts in nest box studies of birds. Oikos. 1989;56(3):421–423. [Google Scholar]
- Møøller AP, Allander R, Dufva R. Fitness effects of parasites on passerine birds: a review. In: Blondel J., Gosler A., Lebreton J.D., McCleery RH, editors. Population biology of passerine birds: an integrated approach. Springer-Verlag; 1990. [Google Scholar]
- Møøller AP. Parasitism and the evolution of host life history. In: Clayton D.H., Moore J, editors. Host-parasite evolution: general principles and avian models. Oxford University Press; 1997. [Google Scholar]
- Philips JR, Fain A. Acarine symbionts of louseflies (Diptera: Hippoboscidae). Acarologia. 1991;32(4):377–384. [Google Scholar]
- Popov AV. The life-cycle of the louse flies Lipoptena cervi L. and Stenepteryx hirundinis L. (Diptera, Hippoboscidae). Entomologicheskoe obozrenie. 1965;44:573–583. (In Russian) [Google Scholar]
- Roulin A, Brinkhof MWG, Bize P, Richner H, Jungi TW, Bavoux C, Boileau N, Burneleau G. Which Chick Is Tasty to Parasites? The Importance of Host Immunology vs. Parasite Life History. The Journal of Animal Ecology. 2003;72:75–81. [Google Scholar]
- Saino N, Calza S, Møøller AP. Effects of a dipteran ectoparasite on immune response and growth trade-offs in barn swallow (Hirundo rustica) nestlings. Oikos. 1998;81:217–228. [Google Scholar]
- Senar JC, Copete JL, Domenech J, Von Walter G. Prevalence of louse flies Diptera: Hippoboscidae parasitizing a cardueline finch and its effects on body condition. Ardea. 1994;82(1):157–160. [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology and Evolution. 1996;11(8):317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Small RW. A review of Melophagus ovinus (L.), the Sheep Ked. Veterinary Parasitology. 2005;130(1–2):141–155. doi: 10.1016/j.vetpar.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Soulsby E.J.L. Helminths, Arthropods, and Protozoa of Domesticated Animals. Williams and Wilkins Co; 1968. [Google Scholar]
- Summers RW. On the ecology of Crataerina hirundinis (Diptera; Hippoboscidae) in Scotland. Journal of Zoology, London. 1975;175:557–570. [Google Scholar]
- Tella JL, Gortáázar C, Gajon A, Osáácar JJ. Apparent lack of effects of a high louse fly infestation (Diptera, Hippoboscidae) on adult colonial Alpine Swifts. Ardea. 1995;83(2):435–439. [Google Scholar]
- Tella JL, Gajóón A, Gortáázar C, Osáácar JJ. High host specificity of Crataerina melba (Diptera, Hippoboscidae) in a mixed colony of birds. The Journal of Parasitology. 1998;84(1):198–200. [PubMed] [Google Scholar]
- Tella JL, Jovani R. Sources of variability in aggregation and sex ratios of Crataerina melba (Diptera: Hippoboscidae) among adult colonial alpine swifts. Journal of Parasitology. 2000;86(5):933–938. doi: 10.1645/0022-3395(2000)086[0933:SOVIAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Tompkins DM, Jones T, Clayton DH. Effect of vertically transmitted ectoparasites on the reproductive success of Swifts (Apus apus). Functional Ecology. 1996;10(6):733–740. [Google Scholar]
- Walker MD, Rozman J, Witte K. Brutkolonie des Mauerseglers (Apus apus) in einer Autobrüüke. Vogelwarte. 2009;47:41–43. [Google Scholar]
- Yuval B. Mating systems of blood feeding flies. Annual Review of Entomology. 1996;51:413–440. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]
- Zumpt F. The arthropod parasites of vertebrates in Africa south of the Sahara. 3 Insecta excl. Phthiraptera. Publications of the South African Institute for Medical Research. 1966;13:1–283. [Google Scholar]