Abstract
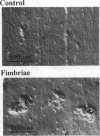
Our previous study demonstrated that Porphyromonas gingivalis fimbriae induce the expression of interleukin-1, a potent bone-resorbing cytokine, in macrophages. This demonstration suggested to use the possibility that the fimbriae may stimulate bone resorption via the generation of an inflammatory cytokine(s). The present study was performed to test this suggestion. The bone-resorbing activity was evaluated by measuring the area of resorption lacunae on bone slices incubated with calvarial bone cells taken from 14-day-old mouse embryos. Fimbriae at 0.5 micrograms of protein per ml stimulated the bone-resorbing activity significantly, and the effect was dose and treatment time dependent. Since it is well known that interleukin-1 and granulocyte macrophage colony-stimulating factor induce differentiation of osteoclast lineage cells, we examined the involvement of these cytokines in fimbria-stimulated bone resorption. Fimbria-stimulated bone resorption was abolished significantly by antisera against both cytokines. We observed by Northern (RNA) blot assay that both cytokine genes were markedly expressed in the fimbria-treated calvarial bone cells. Our present data demonstrate that P. gingivalis fimbriae stimulate bone resorption in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agace W., Hedges S., Andersson U., Andersson J., Ceska M., Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993 Feb;61(2):602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano S., Hanazawa S., Hirose K., Ohmori Y., Kitano S. Stimulatory effect on bone resorption of interleukin-1-like cytokine produced by an osteoblast-rich population of mouse calvarial cells. Calcif Tissue Int. 1988 Aug;43(2):88–91. doi: 10.1007/BF02555152. [DOI] [PubMed] [Google Scholar]
- Amano S., Hanazawa S., Kawata Y., Ohta K., Kitami H., Kitano S. An assay system utilizing devitalized bone for assessment of differentiation of osteoclast progenitors. J Bone Miner Res. 1992 Mar;7(3):321–328. doi: 10.1002/jbmr.5650070312. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chambers T. J. The regulation of osteoclastic development and function. Ciba Found Symp. 1988;136:92–107. doi: 10.1002/9780470513637.ch7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Klausen B., Genco R. J. Immunization with fimbrial protein and peptide protects against Porphyromonas gingivalis-induced periodontal tissue destruction. Adv Exp Med Biol. 1992;327:255–262. doi: 10.1007/978-1-4615-3410-5_27. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Klausen B., Sojar H. T., Bedi G. S., Sfintescu C., Ramamurthy N. S., Golub L. M., Genco R. J. Immunization with Porphyromonas (Bacteroides) gingivalis fimbriae protects against periodontal destruction. Infect Immun. 1992 Jul;60(7):2926–2935. doi: 10.1128/iai.60.7.2926-2935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Hofstetter W., Guenther H. L., Fleisch H. Production of granulocyte-macrophage (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) by rat clonal osteoblastic cell population CRP 10/30 and the immortalized cell line IRC10/30-myc1 stimulated by tumor necrosis factor alpha. Endocrinology. 1991 Feb;128(2):661–667. doi: 10.1210/endo-128-2-661. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Hanazawa S., Hirose K., Ohmori Y., Amano S., Kitano S. Bacteroides gingivalis fimbriae stimulate production of thymocyte-activating factor by human gingival fibroblasts. Infect Immun. 1988 Jan;56(1):272–274. doi: 10.1128/iai.56.1.272-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Murakami Y., Hirose K., Amano S., Ohmori Y., Higuchi H., Kitano S. Bacteroides (Porphyromonas) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect Immun. 1991 Jun;59(6):1972–1977. doi: 10.1128/iai.59.6.1972-1977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Murakami Y., Takeshita A., Kitami H., Ohta K., Amano S., Kitano S. Porphyromonas gingivalis fimbriae induce expression of the neutrophil chemotactic factor KC gene of mouse peritoneal macrophages: role of protein kinase C. Infect Immun. 1992 Apr;60(4):1544–1549. doi: 10.1128/iai.60.4.1544-1549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Takeshita A., Amano S., Semba T., Nirazuka T., Katoh H., Kitano S. Tumor necrosis factor-alpha induces expression of monocyte chemoattractant JE via fos and jun genes in clonal osteoblastic MC3T3-E1 cells. J Biol Chem. 1993 May 5;268(13):9526–9532. [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Effects of interleukin 3 and of granulocyte-macrophage and macrophage colony stimulating factors on osteoclast differentiation from mouse hemopoietic tissue. J Cell Physiol. 1990 Jan;142(1):201–209. doi: 10.1002/jcp.1041420125. [DOI] [PubMed] [Google Scholar]
- Kreft B., Bohnet S., Carstensen O., Hacker J., Marre R. Differential expression of interleukin-6, intracellular adhesion molecule 1, and major histocompatibility complex class II molecules in renal carcinoma cells stimulated with S fimbriae of uropathogenic Escherichia coli. Infect Immun. 1993 Jul;61(7):3060–3063. doi: 10.1128/iai.61.7.3060-3063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo J. A., Sousa S. L., Fonseca J. M., Hock J. M., Medlock E. S. Colony-stimulating factors regulate the development of multinucleated osteoclasts from recently replicated cells in vitro. J Clin Invest. 1987 Jul;80(1):160–164. doi: 10.1172/JCI113042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortie L. A., Harel J., Fairbrother J. M., Dubreuil J. D. Immunodot detection of Escherichia coli heat-stable enterotoxin b by using enhanced chemiluminescence reaction. J Clin Microbiol. 1991 Oct;29(10):2250–2252. doi: 10.1128/jcm.29.10.2250-2252.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Hanazawa S., Nishida K., Iwasaka H., Kitano S. N-acetyl-D-galactosamine inhibits TNF-alpha gene expression induced in mouse peritoneal macrophages by fimbriae of Porphyromonas (Bacteroides) gingivalis, an oral anaerobe. Biochem Biophys Res Commun. 1993 Apr 30;192(2):826–832. doi: 10.1006/bbrc.1993.1489. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Kusumoto Y., Uchida H., Nagashima S., Ogo H., Hamada S. Immunobiological activities of synthetic peptide segments of fimbrial protein from Porphyromonas gingivalis. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1335–1341. doi: 10.1016/s0006-291x(05)81342-7. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Chenu C., Bird A., Mundy G. R., Roodman G. D. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J Bone Miner Res. 1989 Feb;4(1):113–118. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Dewhirst F. E., Peros W. J., Kent R. L., Ago J. M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987 Mar 1;138(5):1464–1468. [PubMed] [Google Scholar]
- Thomson B. M., Mundy G. R., Chambers T. J. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987 Feb 1;138(3):775–779. [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]