Abstract
Objective
To assess the association between food combination and Alzheimer disease (AD) risk. Because foods are not consumed in isolation, dietary pattern (DP) analysis of food combination, taking into account the interactions among food components, may offer methodological advantages.
Design
Prospective cohort study.
Setting
Northern Manhattan, New York, New York.
Patients or Other Participants
Two thousand one hundred forty-eight community-based elderly subjects (aged ≥65 years) without dementia in New York provided dietary information and were prospectively evaluated with the same standardized neurological and neuropsychological measures approximately every 1.5 years. Using reduced rank regression, we calculated DPs based on their ability to explain variation in 7 potentially AD-related nutrients: saturated fatty acids, monounsaturated fatty acids, ω-3 polyunsaturated fatty acids, ω-6 poly-unsaturated fatty acids, vitamin E, vitamin B12, and folate. The associations of reduced rank regression–derived DPs with AD risk were then examined using a Cox proportional hazards model.
Main Outcome Measure
Incident AD risk.
Results
Two hundred fifty-three subjects developed AD during a follow-up of 3.9 years. We identified a DP strongly associated with lower AD risk: compared with subjects in the lowest tertile of adherence to this pattern, the AD hazard ratio (95% confidence interval) for subjects in the highest DP tertile was 0.62 (0.43–0.89) after multivariable adjustment (P for trend=.01). This DP was characterized by higher intakes of salad dressing, nuts, fish, tomatoes, poultry, cruciferous vegetables, fruits, and dark and green leafy vegetables and a lower intake of high-fat dairy products, red meat, organ meat, and butter.
Conclusion
Simultaneous consideration of previous knowledge regarding potentially AD-related nutrients and multiple food groups can aid in identifying food combinations that are associated with AD risk.
Epidemiological evidence linking diet, one of the most important modifiable environmental factors, and risk of AD is rapidly increasing. However, current literature regarding the impact of individual nutrients or food items on AD risk is inconsistent, partly because humans eat meals with complex combinations of nutrients or food items that are likely to be synergistic.1 As an alternative approach, dietary pattern (DP) analysis has emerged in recent years.1,2
One type of approach to derive DPs is a hypothesis-driven, or a priori, approach, which requires prior knowledge of diet and diseases and uses existing scores to indicate a DP. Two cross-sectional studies have used this approach to derive DPs that were linked to cognitive function.3,4 Another example is the Mediterranean diet (MeDi), higher adherence to which we found to be related to a lower risk for AD in the Washington Heights–Inwood Columbia Aging Project (WHICAP) population.5–8 However, there are a few limitations with the MeDi approach: (1) The MeDi does not take advantage of accumulating knowledge on the relation between nutrients and neurodegeneration. (2) Only a limited number of foods (9 food groups) are considered in the MeDi. (3) The multiethnic population of the WHICAP study may not strictly consume foods typical of the Mediterranean countries, and the “MeDi adherence” of our study population may therefore be significantly lower as compared with Mediterranean populations.
The second type of DP-deriving approach is exploratory, or a posteriori, in nature. It uses statistical methods such as principal component analysis to derive DPs. A few cross-sectional studies have applied this approach to examine the associations between DPs and cognitive function.9,10 However, DPs identified by this approach may not be the best predictors of disease risk,2 because this approach is entirely empirical, ignoring key nutrients that are likely to be implied in the physiopathology.
A third option to derive DPs is reduced rank regression (RRR),11 which combines the a priori approach by using prior information of nutrients-disease association existing in the literature and the a posteriori approach by using study-specific data.11 Since biologic knowledge concerning development of a disease is based on the role of nutrients rather than that of foods, DPs derived by using RRR should better clarify the importance of diet in the etiology of diseases than an approach such as the MeDi one. Previous studies have successfully identified RRR-derived DPs that were strongly associated with risks of various diseases, such as diabetes mellitus,12 coronary heart disease,11,13–19 and cancers.20,21 Nevertheless, to our knowledge, the method has yet to be applied in the neurology field.
In the current study, we aimed to identify a combination of foods that may relate to AD risk by applying RRR in dietary data from 2148 elderly subjects without dementia followed up prospectively for approximately 4 years.
METHODS
STUDY POPULATION
The study included participants of 2 related cohorts recruited in 1992 (WHICAP 1992) and 1999 (WHICAP 1999) who were identified from a probability sample of Medicare beneficiaries residing in northern Manhattan, New York.5–8,22 At entry, a physician elicited each subject’s medical and neurological histories and conducted standardized physical and neurological examinations. Each subject also underwent a structured in-person interview including an assessment of health and function and a neuropsychological battery.23 A global summary score on the Clinical Dementia Rating (CDR)24 was assigned. Subjects were followed up at intervals of approximately 1.5 years, repeating the baseline examination and consensus diagnosis.5–8
ALZHEIMER DISEASE DIAGNOSIS
A consensus diagnosis for the presence or absence of dementia was made at a diagnostic conference attended by neurologists and neuropsychologists, using the neuropsychological battery of tests and evidence of cognitive deficit (based on the neuropsychological scores as described earlier), evidence of impairment in social or occupational function (as assessed by the Blessed Dementia Rating Scale, the Schwab and England Activities of Daily Living Scale, and the physician’s assessment), and evidence of cognitive and social/occupational function decline as compared with the past, as required by the Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised).
The type of dementia was subsequently determined. For the diagnosis of probable or possible Alzheimer disease (AD), the criteria of the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association25 were used. Since, according to the criteria, stroke does not preclude the diagnosis of AD (unless cerebrovascular disease is considered the primary cause of the dementia), the diagnosis of AD with concomitant stroke was also assigned. Therefore, dementia cases with cerebrovascular damage could be classified either in the non-AD dementia category or in the AD category.
Dietary data were not available to the consensus panel and were not considered in the diagnostic process.
DIET DATA
Average food consumption over the year before the baseline assessment was obtained using a 61-item version of the Willett semiquantitative food frequency questionnaire (SFFQ) (Channing Laboratory, Cambridge, Massachusetts). Trained interviewers administered the SFFQ in English or Spanish. The SFFQs have been used and validated for the determination of nutrient intake in the elderly26 and WHICAP22 populations. The 61 food items were categorized into 30 food groups based on similarities in food and nutrient composition, and intake (grams per day) of each food group was then calculated by summing the intakes of member food items (eTable 1, http://www.archneurol.com). The daily intake of nutrients was computed by multiplying the consumption frequency of each portion of every food by the nutrient content of the specified portion.27 The nutrient intakes from foods and from supplements were separately estimated, and only the nutrient intake from foods was used in the RRR analysis.
COVARIATES EVALUATION
Information about recruitment cohort, age, sex, education, ethnicity, body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared), and smoking status was obtained from baseline interviews. Caloric intake and alcohol consumption were calculated from the baseline SFFQ. Apolipoprotein E (APOE) ε4 genotype was used as a dichotomous variable: absence vs presence (of either 1 or 2) of ε4 alleles. A modified version5 of the Charlson Comorbidity Index28 (hereafter referred to as the “comorbidity index”) included items for myocardial infarction, congestive heart failure, peripheral vascular disease, hypertension, chronic obstructive pulmonary disease, arthritis, gastrointestinal disease, mild liver disease, diabetes, chronic renal disease, and systemic malignancy from the initial visit. All items received weights of 1, with the exception of chronic renal disease and systemic malignancy, which were weighted 2. The index was treated as a continuous variable.
STATISTICAL ANALYSIS
Reduced Rank Regression
Reduced rank regression determines linear combinations, ie, DP scores, of a set of predicting variables (food groups) by maximizing the explained variation of a set of response variables (nutrients) (eFigure 1).11 In this study, RRR was performed using 30 predetermined food groups (eTable 1) as predicting variables and 7 nutrients, saturated fatty acids (SFA), monounsaturated fatty acids, ω-3 polyunsaturated fatty acids (PUFA), ω-6 PUFA, vitamin E, vitamin B12, and folate, as response variables. The 7 nutrients are most consistently reported to be related to dementia risk according to the literature. Current evidence suggests that an increase of dietary intake of SFA or total fats could have negative effects on cognitive functions,29,30 while increased intake of PUFA30,31 and monounsaturated fatty acids30,31 may be protective against cognitive decline. Higher intakes of vitamin B12,32–35 folate,32–35 and vitamin E36–39 may be related to better cognitive functioning or lower risk of AD in elderly individuals. Both food group and nutrient intakes were adjusted for caloric intake using the regression residual method,40 and their residuals were used in the analysis. Using an alternative method of total caloric intake adjustment (ie, Density Model: food group intakes divided by total caloric intake [grams per kilocalories]), did not change our results (data not shown). For each subject, 7 DP scores, representing 7 mutually uncorrelated DPs, were obtained.11 A higher DP score indicates a stronger adherence of a subject’s diet to the particular DP.
Survival Analyses
To evaluate the association between the extracted DPs and risk of AD, we ran Cox models with AD as the dichotomous outcome, time to event as the time metric, and each of the 7 DP scores as the main predicting variable. The time-to-event variable was time from recording of baseline diet to first visit of AD diagnosis for incident cases or to the time of the last follow-up for noncases. The following variables were adjusted: recruitment cohort, age, sex, ethnicity, education, smoking status, BMI, caloric intake, comorbidity index, and APOE ε4 genotype. Effect modification by these covariates was tested by including an interaction term into the Cox models. All variables were used as time-constant covariates.
Supplementary Analyses
Current evidence suggests that moderate alcohol drinking may be protective against incident dementia or delay age-associated cognitive decline.22 Additionally, nutrient levels are also affected by subjects’ intake of supplements. We therefore adjusted for alcohol drinking or supplements intake of nutrients to control confounding effects by these factors.
Among 2148 subjects without dementia (CDR≤0.5)41 at baseline, 312 (15%) had a CDR of 0.5, which may be an index of early subclinical disease. To increase our confidence that the degree of adherence to DPs was not affected by the early subclinical dementia process, we performed analysis by excluding subjects who were followed up for less than 2 years and adjusting for baseline CDR status.
To overcome the limitation of the subjectiveness of nutrients selection and to test the robustness of the results, we performed RRR analyses by including into the model vitamin C, β-carotene, or both and obtained 3 sets of new DPs. The resulting 3 sets of DPs were then compared with the original set of 7 DPs from the original model with 7 nutrients. These 2 nutrients were associated with risk of AD as suggested in the literature,36,38,39,42,43 although not always consistently.44–46
In other exploratory models, we repeated the analyses excluding subjects who were diagnosed as having AD with concomitant stroke (ie, using only AD without stroke as the outcome). We also repeated the analyses including probable AD cases only.
Stability of RRR-Derived DP Scores
We used generalized estimating equations, with the DP scores as the dependent variable and time (in years) as the predictor, to test whether there were significant changes of RRR-derived DP scores over time for a subset of subjects with more than 1 dietary assessment.5 We assessed the stability of RRR-derived DP scores separately for incident cases and for subjects who remained without dementia.
The RRR analyses were performed with SAS 9.1 (SAS Institute Inc, Cary, North Carolina) and all the other analyses were performed using SPSS 12.0 (SPSS Inc, Chicago, Illinois).
RESULTS
PARTICIPANT CHARACTERISTICS
A total of 3436 among the initial 4166 subjects were dementia-free at baseline. Because the dietary assessment was added after initiation of the study, dietary information was missing for 527 subjects.5 An additional 627 subjects were excluded because follow-up was not available for them, among which 101 subjects died within 1.5 years from baseline visit and 526 subjects were lost to follow-up.5 Also excluded were 102 subjects who had incomplete dietary information prohibiting calculation of RRR-derived DP scores and 32 subjects who developed dementia other than AD during the follow-up period. Thus, the analytic sample comprised 2148 subjects.
Compared with subjects remaining in the final analysis (N=2148), subjects with missing follow-up data (n=526) were slightly younger (76.2 vs 77.2 years; P=.004), less likely to be smokers (9.1% vs 12.5%; P=.03), and less likely to be moderate alcohol drinkers (25.7% vs 32.3%; P=.003) and had lower education (9.6 vs 10.0 years; P=.04), higher caloric intake (1488.4 vs 1426.3 kcal/d; P=.02), and higher BMI (28.0 vs 27.3; P=.03).
After an average follow-up of 3.96 years (SD, 3.0 years; range, 0.14–13.9 years), 253 incident cases of AD were identified. Compared with subjects who remained without dementia, incident AD cases were older (P<.001), were less educated (P<.001), had a lower BMI (P=.01), were more likely to be Hispanic and less likely to be white (P<.001), and were less likely to be moderate alcohol drinkers (P<.001) (eTable 2).
DP SCORES AND RISK OF AD
The 7 DP scores, taken together, explained 76.8% and 29.5% of the total variation in nutrients and foods intakes, respectively (Table 1). The crude hazard ratios (95% confidence interval; P trend) for subjects in the highest tertile compared with those in the lowest tertile for DP scores 1 through 7, respectively, were as follows: 1.06 (0.78–1.45; .68), 0.54 (0.39–0.75; <.001), 1.10 (0.81–1.47; .58), 1.26 (0.94–1.70; .13), 1.16 (0.86–1.57; .34), 0.94 (0.69–1.27; .66), and 0.96 (0.72–1.29; .82).
Table 1.
Explained Variations of Nutrients and Food Groups by Extracted DPs and Correlations Between Nutrients and DP Scores
| Nutrients | DP 1 | DP 2 | DP 3 | DP 4 | DP 5 | DP 6 | DP 7 | Total |
|---|---|---|---|---|---|---|---|---|
| Explained % of Variation in Nutrients and Food Groups | ||||||||
| SFA | 47.87 | 19.37 | 1.56 | 4.98 | 6.51 | 0.02 | 1.56 | 81.87 |
| MUFA | 70.62 | 0.59 | 1.91 | 7.97 | 1.69 | 0.00 | 2.39 | 85.18 |
| ω-3 PUFA | 9.27 | 36.34 | 1.96 | 5.09 | 13.11 | 0.08 | 0.48 | 66.33 |
| ω-6 PUFA | 23.07 | 48.75 | 1.26 | 0.11 | 3.34 | 2.53 | 1.10 | 80.16 |
| Vitamin E | 0.03 | 13.85 | 1.51 | 2.36 | 1.06 | 10.16 | 0.22 | 29.18 |
| Vitamin B12 | 0.17 | 6.05 | 84.92 | 6.53 | 0.71 | 0.00 | 0.00 | 98.39 |
| Folate | 52.14 | 5.30 | 13.24 | 23.81 | 1.61 | 0.62 | 0.04 | 96.76 |
| Explained proportion of variation of all nutrients | 29.02 | 18.61 | 15.20 | 7.26 | 4.00 | 1.91 | 0.83 | 76.84 |
| Explained proportion of variation of all food groups | 6.20 | 5.17 | 4.02 | 3.58 | 3.34 | 3.95 | 3.26 | 29.52 |
| Pearson Correlation Coefficients Between Nutrients and Extracted DPs | ||||||||
| SFA | 0.69a | −0.44a | 0.12a | 0.22a | 0.26a | 0.01 | 0.12a | |
| MUFA | 0.84a | −0.08 | 0.14a | 0.28a | −0.13a | 0.00 | −0.15a | |
| ω-3 PUFA | 0.30a | 0.60a | 0.14a | −0.23a | 0.36a | 0.03 | −0.07 | |
| ω-6 PUFA | 0.48a | 0.70a | 0.11a | 0.03 | −0.18a | −0.16a | 0.11a | |
| Vitamin E | 0.02 | 0.37a | 0.12a | 0.15a | −0.10a | 0.32a | 0.05 | |
| Vitamin B12 | −0.04 | −0.25a | 0.92a | −0.26a | −0.08a | 0.00 | 0.01 | |
| Folate | −0.72a | 0.23a | 0.36a | 0.49a | 0.13a | −0.08 | −0.02 | |
Abbreviations: DP, dietary pattern; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
P<.001.
Our subsequent analyses focused on DP score 2, the only DP that was significantly associated with AD risk. The DP score 2 was normally distributed with mean (SD) of 0.001 (1.06). A high DP score 2 reflected a diet rich in ω-3 PUFA, ω-6 PUFA, vitamin E, and folate (positively correlated, P<.001), but poor in SFA and vitamin B12 (negatively correlated, P<.001) (Table 1). The DP score 2 was positively correlated with intakes of salad dressing, nuts, fish, tomatoes, poultry, cruciferous vegetables, fruits, and dark and green leafy vegetables (factor loadings ≥0.15) and negatively correlated with intakes of high-fat dairy, red meat, organ meat, and butter (factor loadings ≤−0.15) (eTable 3). Subjects who were older, less educated, and current smokers tended to adhere less to DP 2. Hispanic individuals adhered less than white and black individuals to DP 2 (P=.02). Women tended to adhere more than men to DP 2 (P=.05) (Table 2).
Table 2.
Characteristics of the Study Population by Tertiles of DP Score 2
| No. (%) |
|||||
|---|---|---|---|---|---|
| Total (N=2148) | Lowest Tertilea (n=749) | Middle Tertile (n=717) | Highest Tertile (n=682) | P Valueb | |
| Cut points of DP score 2 | −3.71 to −0.39 | −0.39 to 0.36 | 0.37 to 16.3 | ||
| Age at entry, y, mean (SD) | 77.2 (6.6) | 77.4 (6.7) | 77.6 (6.6) | 76.5 (6.3) | .02 |
| Female | 1457 (68) | 485 (65) | 497 (69) | 475 (70) | .05 |
| Education, y, mean (SD) | 10.0 (4.8) | 9.5 (4.7) | 9.8 (4.6) | 10.8 (4.8) | <.001 |
| Ethnicity | |||||
| White | 596 (28) | 200 (27) | 188 (26) | 208 (31) | .02 |
| Black | 693 (32) | 218 (29) | 252 (35) | 223 (33) | |
| Hispanic | 823 (38) | 320 (43) | 266 (37) | 237 (35) | |
| Other | 36 (2) | 11 (2) | 11 (2) | 14 (2) | |
| Presence of APOE ε4 allele | 508 (27) | 193 (29) | 174 (28) | 141 (25) | .07 |
| Current smoker | 268 (13) | 122 (16) | 79 (11) | 67 (10) | <.001 |
| Comorbidity index, mean (SD) | 1.9 (1.4) | 1.83 (1.4) | 1.99 (1.4) | 1.96 (1.5) | .09 |
| Caloric intake, kcal/d, mean (SD) | 1426 (515) | 1495 (523) | 1320 (492) | 1460 (512) | .14 |
| BMI, mean (SD) | 27.4 (5.5) | 27.1 (5.3) | 27.5 (5.8) | 27.5 (5.4) | .23 |
| Moderate alcohol drinking | 693 (32) | 244 (33) | 209 (29) | 240 (35) | .35 |
| Incident AD cases | 253 (12) | 117 (16) | 86 (12) | 50 (7) | <.001 |
Abbreviations: AD, Alzheimer disease; APOE, apolipoprotein E; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DP, dietary pattern.
The tertiles were defined based on the distribution of the DP score 2 among participants who remained without dementia during the follow-up.
P values for trend from logistic regression for binary variables and from linear regression models for continuous variables, with tertiles of the DP score entering the models as an ordinal variable. P value for the categorical variable (ie, ethnicity) was from χ2 test.
The association between DP 2 and AD risk (model 1, Table 3) (Figure) was essentially unchanged after adjustment for demographic factors including recruitment cohort, age, education, ethnicity, and sex in a second model (model 2, Table 3). Additional adjustment for smoking status, BMI, caloric intake, comorbidity index, and APOE ε4 genotype (model 3) only slightly attenuated the AD risk: compared with subjects in the lowest DP score 2 tertile, subjects in the middle and highest tertiles, respectively, had 19% and 38% less risk for AD (P trend=.01) (Table 3). No effect modification by any covariate was found.
Table 3.
HRs of Incident AD Associated With DP Score 2
| Modela | No. of Cases/Total at Baseline | DP Score 2 Tertileb | HR (95% CI) | P Value | P Trendc |
|---|---|---|---|---|---|
| Model 1 | 253/2148 | Lowest | 1 [Reference] | ||
| Middle | 0.73 (0.56–0.97) | .03 | |||
| Highest | 0.54 (0.39–0.75) | <.001 | <.001 | ||
| Model 2 | 251/2141 | Lowest | 1 [Reference] | ||
| Middle | 0.72 (0.54–0.95) | .02 | |||
| Highest | 0.59 (0.42–0.82) | .002 | .001 | ||
| Model 3 | 211/1691 | Lowest | 1 [Reference] | ||
| Middle | 0.81 (0.59–1.12) | .20 | |||
| Highest | 0.62 (0.43–0.89) | .01 | .01 | ||
| Supplementary Analysis | |||||
| Model 2 and alcohol | 251/2141 | Lowest | 1 [Reference] | ||
| Middle | 0.70 (0.52–0.93) | .01 | |||
| Highest | 0.59 (0.42–0.82) | .002 | .002 | ||
| Model 2 and nutrient supplements | 251/2141 | Lowest | 1 [Reference] | ||
| Middle | 0.72 (0.54–0.96) | .03 | |||
| Highest | 0.60 (0.43–0.85) | .003 | .002 | ||
| Model 2 and alcohol and nutrient supplements | 251/2141 | Lowest | 1 [Reference] | ||
| Middle | 0.70 (0.53–0.93) | .02 | |||
| Highest | 0.60 (0.43–0.85) | .003 | .002 | ||
| Model 2 and baseline CDRd | 204/1758 | Lowest | 1 [Reference] | ||
| Middle | 0.66 (0.48–0.91) | .01 | |||
| Highest | 0.64 (0.44–0.92) | .02 | .007 | ||
Abbreviations: AD, Alzheimer disease; CI, confidence interval; CDR, Clinical Dementia Rating; DP, dietary pattern; HR, hazard ratio.
Model 1: unadjusted. Model 2: adjusted for recruitment cohort, age, education, ethnicity, and sex. Model 3: adjusted for covariates in model 2 and smoking status, body mass index, caloric intake, comorbidity index, and APOE ε4 genotype.
The tertiles were defined based on the distribution of the DP score 2 among participants who remained without dementia during the follow-up.
P trend was obtained by entering the tertile terms as an ordinal variable in the Cox model.
Limited to subjects with more than 2 years of follow-up.
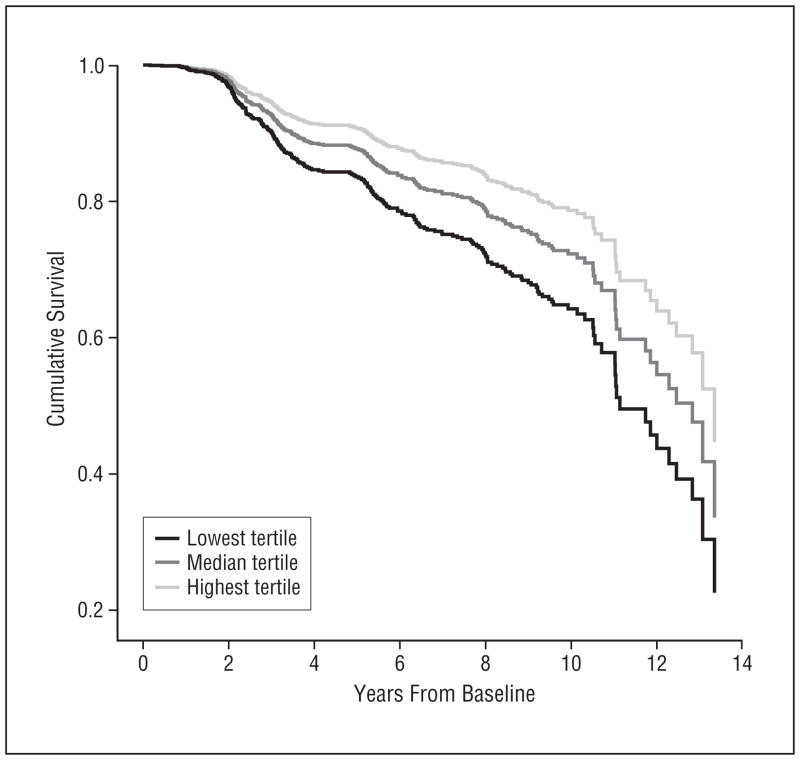
Figure.
Survival curves based on Cox analysis comparing cumulative Alzheimer disease incidence in subjects belonging to each dietary pattern (DP) score 2 tertile (P for trend <.001). Lowest tertile (black line) corresponds to the lowest adherence to DP 2; median tertile (dark-gray line), to median adherence; and highest tertile (light-gray line), to the highest adherence. The Figure was derived from a crude model that used all subjects (N=2148).
SUPPLEMENTARY ANALYSES
Adding alcohol, nutrient supplements, or both in model 2 did not materially change the associations (Table 3). Limiting analysis to the 1758 subjects with more than 2 years of follow-up, and adjusting baseline CDR in addition to all the covariates in model 2, the hazard ratios (95% confidence interval; P value) for the middle and highest tertiles were respectively 0.66 (0.48–0.91; .01) and 0.64 (0.44–0.92; .02) (P trend=.007) compared with the lowest tertile (Table 3).
In RRR analysis considering additional nutrients, we were able to extract additional 3 DPs that were similar to DP 2 in terms of characterization of food consumption as well as the associations with AD risk (eTable 4). None of the other extracted DPs was significantly associated with the risk of AD in any of the models (data not shown).
We repeated the analyses considering only 215 AD cases without concomitant stroke (ie, excluding AD with coexisting stroke, n=38). The results were essentially unchanged. In a model adjusted for recruitment cohort, age, education, ethnicity, and sex, compared with subjects in the lowest tertile of DP score 2, AD hazard ratios (95% confidence interval) for subjects in the middle and highest DP score 2 tertiles were 0.76 (0.56–1.03) and 0.58 (0.40–0.84), respectively (P for trend=.003). None of the other DPs were significantly associated with AD risk (data not shown).
When limiting analysis to probable AD only (a total of 184 patients), after adjusting for recruitment cohort, age, education, ethnicity, and sex, compared with subjects in the lowest tertile of adherence to this pattern, the probable AD hazard ratios (95% confidence interval) for subjects in the middle and highest DP tertiles were 0.68 (0.48–0.97) and 0.64 (0.44–0.95), respectively (P for trend=.02).
DP SCORE 2 STABILITY
There were 1104 subjects with 2 or more dietary assessments who remained without dementia during follow-up. The mean interval between the first and second available dietary assessments was 5.3 years (SD, 2.1 years; range, 1.0–15.4 years). The DP score 2 did not change over time (β=−0.02; P=.11).
There were 120 subjects with 2 or more dietary assessments who developed incident AD during follow-up. The mean interval between the first and second available dietary assessments was 6.8 years (SD, 2.8 years; range, 1.8–15.5 years). The DP score 2 did not change over time (β=−0.02; P=.41).
COMMENT
In this prospective study, we identified a DP that explained variation of AD-related nutrients and was strongly protective against the development of AD, even after controlling for multiple covariates. This DP reflected a diet rich in ω-3 PUFA, ω-6 PUFA, vitamin E, and folate, but with lower SFA and vitamin B12. Furthermore, dietary habits of subjects adhering more to this DP were characterized as high intake of salad dressing, nuts, fish, tomatoes, poultry, cruciferous vegetables, fruits, and dark and green leafy vegetables and low intake of high-fat dairy, red meat, organ meat, and butter.
We previously reported that higher adherence to the MeDi was associated with reduced risk of AD in this WHICAP population.5,6 However, this multiethnic population may not strictly consume foods typical of the ones consumed in Mediterranean countries. Hence, one goal of the present study was to see whether there are “naturally existing” DPs in our population that may also be associated with AD risk. Interestingly, the identified protective DP 2 was very similar to the MeDi,5–7,47 and they are correlated with each other (Pearson r=0.35; P<.001). Nevertheless, from another point of view, this means only about 12% of DP 2’s variance was shared by the MeDi, indicating that despite some overlap, important new information was obtained in the current study. For example, rather than treating each food group equally in MeDi, the factor loadings of food–DP 2 help to identify the relative importance of each food group, thus providing more specific information.
Certain clarifications are needed in interpreting the results of the RRR method. Despite the fact that DP 1 explained most of the variation of nutrients, it was not associated with risk of AD. Previous literature shows that it is not always the DP that explains most of the variance that is predictive of disease risk.11 In addition, the null association of DP 1 with AD might be due to the fact that DP 1 was positively correlated with both SFA and monounsaturated fatty acids, which might have opposite effects on AD risk. As a result, higher adherence to DP 1 might result in consumption of nutrients with opposing effects regarding the outcome. These results also indicated that a high or low intake of a single food/nutrient might reveal little information without taking into account other foods/nutrients. Another example is the low intake of vitamin B12 in the protective DP 2, which might lead to an interpretation of vitamin B12 as an independent risk factor for AD. However, source foods of vitamin B12 like meat and dairy products may also contain a high level of SFA, which is a potential AD risk factor.30 In addition, DP 2 only explained a very small proportion (6.05%) of variation in vitamin B12. Therefore, DPs potentially important for disease risk may not be the ones that explain most of the variance of a single nutrient but the ones that represent the optimal combination of nutrients (ie, simultaneous higher and lower quantities of various nutrients that might offer the optimal biological synergy in relation to disease risk). In fact, all these results reinforce the importance of studying DPs rather than individual foods or nutrients. The DP analysis is preferable also because of some of its advantages over the analysis of single nutrients or food items. The effect of a single nutrient or food item may be too small to detect. Indeed, none of the nutrients was significantly associated with AD risk in a fully adjusted model (data not shown). Or, a statistically significant association might be simply found by chance alone because of multiple comparisons of many nutrients/foods or the interactions among them.2
The AD risk reduction role played by DP 2 is in general in the expected direction as suggested by the associations between AD risk and these individual nutrients,29–39 except for vitamin B12. Furthermore, the selected 7 nutrients (SFA, monounsaturated fatty acids, ω-3 PUFA, ω-6 PUFA, vitamin E, vitamin B12, and folate) reflected multiple pathways in the pathogenesis of AD. For example, vitamin B12 and folate are homocysteine-related vitamins that may have an impact on AD via their ability of reducing circulating homocysteine levels,48 vitamin E might prevent AD via its strong antioxidant effect,48 and fatty acids may be related to dementia and cognitive function through atherosclerosis, thrombosis, or inflammation via an effect on brain development and membrane functioning or via accumulation of β-amyloid.49 Thus, DP 2, reflecting a diet rich in ω-3 PUFA, ω-6 PUFA, vitamin E, and folate, but poor in SFA and vitamin B12, may have the protective effect on AD involving all these pathways.
Our study has several limitations. First, we used a single measurement of the diet, which might not capture the long-term diet habit of the subjects. However, our analysis showed that DP 2 was quite stable over a period of nearly 6 years. Second, excluding subjects from the final analysis because of loss to follow-up or missing data might have introduced selection bias. Third, although DP 2 was still associated with a reduced risk of AD after controlling for multiple socioeconomic factors, we could not completely rule out the possibility of this association being due to residual confounding. Finally, the associations between DP 2 and incident AD did not change materially after adjusting for CDR at baseline and excluding subjects with a short period of follow-up, but bias due to preclinical disease cannot be completely excluded.
Our study has many advantages. Dietary data were collected using a previously validated instrument.22 The prospective study design reduces the possibility of recall bias. The diagnosis of AD was based on comprehensive clinical and neuropsychological assessment and standard research criteria. Measures for multiple potential AD risk factors have been carefully recorded and adjusted for in the analyses. The study participants were followed up at relatively short intervals.
In conclusion, we identified a DP that was strongly protective against the development of AD. The results of the current study indicate that higher consumption of certain foods (salad dressing, nuts, fish, tomatoes, poultry, cruciferous vegetables, fruits, dark and green leafy vegetables) and lower of others (high-fat dairy, red meat, organ meat, and butter) may be associated with a decreased risk of developing AD via a more favorable profile of nutrients (ie, lower ingestion of SFA and higher ingestion of PUFA, vitamin E, and folate). Our findings provide support for further exploration of food combination–based dietary behavior for the prevention of this important public health problem.
Acknowledgments
Funding/Support: This work was supported by federal National Institute on Aging grants AG028506 and P01-AG07232.
Footnotes
Financial Disclosure: None reported.
Online-Only Material: The eTables and eFigure are available at http://www.archneurol.com.
Author Contributions: Study concept and design: Gu, Nieves, Stern, and Scarmeas. Acquisition of data: Scarmeas. Analysis and interpretation of data: Gu, Nieves, Luchsinger, and Scarmeas. Drafting of the manuscript: Gu and Scarmeas. Critical revision of the manuscript for important intellectual content: Gu, Nieves, Stern, Luchsinger, and Scarmeas. Statistical analysis: Gu, Nieves, and Scarmeas. Obtained funding: Luchsinger and Scarmeas. Administrative, technical, and material support: Stern and Scarmeas. Study supervision: Scarmeas.
References
- 1.Jacobs DR, Jr, Gross MD, Tapsell LC. Food synergy: an operational concept for understanding nutrition. Am J Clin Nutr. 2009;89(5):1543S–1548S. doi: 10.3945/ajcn.2009.26736B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Huijbregts PP, Feskens EJ, Rasanen L, et al. Dietary patterns and cognitive function in elderly men in Finland, Italy and The Netherlands. Eur J Clin Nutr. 1998;52(11):826–831. doi: 10.1038/sj.ejcn.1600654. [DOI] [PubMed] [Google Scholar]
- 4.Corrêa Leite ML, Nicolosi A, Cristina S, Hauser WA, Nappi G. Nutrition and cognitive deficit in the elderly: a population study. Eur J Clin Nutr. 2001;55(12):1053–1058. doi: 10.1038/sj.ejcn.1601270. [DOI] [PubMed] [Google Scholar]
- 5.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59(6):912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66(2):216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarmeas N, Luchsinger JA, Mayeux R, Stern Y. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007;69(11):1084–1093. doi: 10.1212/01.wnl.0000277320.50685.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63(12):1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samieri C, Jutand MA, Feart C, Capuron L, Letenneur L, Barberger-Gateau P. Dietary patterns derived by hybrid clustering method in older people: association with cognition, mood, and self-rated health. J Am Diet Assoc. 2008;108(9):1461–1471. doi: 10.1016/j.jada.2008.06.437. [DOI] [PubMed] [Google Scholar]
- 10.Akbaraly TN, Singh-Manoux A, Marmot MG, Brunner EJ. Education attenuates the association between dietary patterns and cognition. Dement Geriatr Cogn Disord. 2009;27(2):147–154. doi: 10.1159/000199235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann K, Schulze MB, Schienkiewitz A, Nothlings U, Boeing H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol. 2004;159(10):935–944. doi: 10.1093/aje/kwh134. [DOI] [PubMed] [Google Scholar]
- 12.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82(3):675–684. doi: 10.1093/ajcn.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weikert C, Hoffmann K, Dierkes J, et al. A homocysteine metabolism-related dietary pattern and the risk of coronary heart disease in two independent German study populations. J Nutr. 2005;135(8):1981–1988. doi: 10.1093/jn/135.8.1981. [DOI] [PubMed] [Google Scholar]
- 14.McNaughton SA, Mishra GD, Brunner EJ. Food patterns associated with blood lipids are predictive of coronary heart disease: the Whitehall II study. Br J Nutr. 2009;102(4):619–624. doi: 10.1017/S0007114509243030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Nettleton JA, Bertoni AG, Bluemke DA, Lima JA, Szklo M. Dietary pattern, the metabolic syndrome, and left ventricular mass and systolic function: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90(2):362–368. doi: 10.3945/ajcn.2009.27538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drogan D, Hoffmann K, Schulz M, Bergmann MM, Boeing H, Weikert C. A food pattern predicting prospective weight change is associated with risk of fatal but not with nonfatal cardiovascular disease. J Nutr. 2007;137(8):1961–1967. doi: 10.1093/jn/137.8.1961. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann K, Zyriax BC, Boeing H, Windler E. A dietary pattern derived to explain biomarker variation is strongly associated with the risk of coronary artery disease. Am J Clin Nutr. 2004;80(3):633–640. doi: 10.1093/ajcn/80.3.633. [DOI] [PubMed] [Google Scholar]
- 18.Nettleton JA, Steffen LM, Schulze MB, et al. Associations between markers of subclinical atherosclerosis and dietary patterns derived by principal components analysis and reduced rank regression in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2007;85(6):1615–1625. doi: 10.1093/ajcn/85.6.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiBello JR, Kraft P, McGarvey ST, Goldberg R, Campos H, Baylin A. Comparison of 3 methods for identifying dietary patterns associated with risk of disease. Am J Epidemiol. 2008;168(12):1433–1443. doi: 10.1093/aje/kwn274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCann SE, McCann WE, Hong CC, et al. Dietary patterns related to glycemic index and load and risk of premenopausal and postmenopausal breast cancer in the Western New York Exposure and Breast Cancer Study. Am J Clin Nutr. 2007;86(2):465–471. doi: 10.1093/ajcn/86.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nöthlings U, Murphy SP, Wilkens LR, et al. A food pattern that is predictive of flavonol intake and risk of pancreatic cancer. Am J Clin Nutr. 2008;88(6):1653–1662. doi: 10.3945/ajcn.2008.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52(4):540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 23.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population: development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49(5):453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 24.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Smith W, Mitchell P, Reay EM, Webb K, Harvey PW. Validity and reproducibility of a self-administered food frequency questionnaire in older people. Aust N Z J Public Health. 1998;22(4):456–463. doi: 10.1111/j.1467-842x.1998.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 27.Willett WC, editor. Nutritional Epidemiology. 2. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42(5):776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 30.Morris MC, Evans DA, Bienias JL, et al. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60(2):194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 31.Solfrizzi V, Colacicco AM, D’Introno A, et al. Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol Aging. 2006;27(11):1694–1704. doi: 10.1016/j.neurobiolaging.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Bryan J, Calvaresi E. Associations between dietary intake of folate and vitamins B-12 and B-6 and self-reported cognitive function and psychological well-being in Australian men and women in midlife. J Nutr Health Aging. 2004;8(4):226–232. [PubMed] [Google Scholar]
- 33.La Rue A, Koehler KM, Wayne SJ, Chiulli SJ, Haaland KY, Garry PJ. Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. Am J Clin Nutr. 1997;65(1):20–29. doi: 10.1093/ajcn/65.1.20. [DOI] [PubMed] [Google Scholar]
- 34.Luchsinger JA, Tang MX, Miller J, Green R, Mayeux R. Higher folate intake is related to lower risk of Alzheimer’s disease in the elderly. J Nutr Health Aging. 2008;12(9):648–650. doi: 10.1007/BF03008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corrada MM, Kawas C, Hallfrisch J, Muller D, Brookmeyer R. Reduced risk of Alzheimer’s disease with high folate intake: the Baltimore Longitudinal Study of Aging. Alzheimers Dement. 2005;1(1):11–18. doi: 10.1016/j.jalz.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masaki KH, Losonczy KG, Izmirlian G, et al. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology. 2000;54(6):1265–1272. doi: 10.1212/wnl.54.6.1265. [DOI] [PubMed] [Google Scholar]
- 37.Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287(24):3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 38.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287(24):3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 39.Zandi PP, Anthony JC, Khachaturian AS, et al. Cache County Study Group. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61(1):82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 40.Willett W, Stampfer M. Implications of total energy intake for epidemiological analyses. In: Willett W, editor. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998. pp. 273–301. [Google Scholar]
- 41.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24(4):637–639. [PubMed] [Google Scholar]
- 42.Paleologos M, Cumming RG, Lazarus R. Cohort study of vitamin C intake and cognitive impairment. Am J Epidemiol. 1998;148(1):45–50. doi: 10.1093/oxfordjournals.aje.a009559. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Shinto L, Connor WE, Quinn JF. Nutritional biomarkers in Alzheimer’s disease: the association between carotenoids, n-3 fatty acids, and dementia severity. J Alzheimers Dis. 2008;13(1):31–38. doi: 10.3233/jad-2008-13103. [DOI] [PubMed] [Google Scholar]
- 44.Laurin D, Masaki KH, Foley DJ, White LR, Launer LJ. Midlife dietary intake of antioxidants and risk of late-life incident dementia: the Honolulu-Asia Aging Study. Am J Epidemiol. 2004;159(10):959–967. doi: 10.1093/aje/kwh124. [DOI] [PubMed] [Google Scholar]
- 45.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60(2):203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 46.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 47.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 48.Luchsinger JA, Noble JM, Scarmeas N. Diet and Alzheimer’s disease. Curr Neurol Neurosci Rep. 2007;7(5):366–372. doi: 10.1007/s11910-007-0057-8. [DOI] [PubMed] [Google Scholar]
- 49.Kalmijn S. Fatty acid intake and the risk of dementia and cognitive decline: a review of clinical and epidemiological studies. J Nutr Health Aging. 2000;4(4):202–207. [PubMed] [Google Scholar]