Abstract
Safe water storage and hand hygiene have been shown to reduce fecal contamination and improve health in experimental settings; however, triggering and sustaining such behaviors is challenging. This study investigates the extent to which personalized information about Escherichia coli contamination of stored water and hands influenced knowledge, reported behaviors, and subsequent contamination levels among 334 households with less than 5-year-old children in peri-urban Dar es Salaam, Tanzania. One-quarter of the study participants received information about strategies to reduce risk of water- and sanitation-related illness. Respondents in another three study cohorts received this same information, along with their household's water and/or hand-rinse test results. Findings from this study suggest that additional work is needed to elucidate the conditions under which such testing represents a cost-effective strategy to motivate improved household water management and hand hygiene.
Introduction
A large body of research has shown the health benefits of providing household-level piped water and sanitation services on diarrheal and respiratory illnesses.1–3 For many households in developing countries, however, individual water and sanitation services are out of the reach for the foreseeable future. For example, whereas 87% of the world's population is classified as having access to “improved water supply services” by the World Health Organization, only 54% of these have piped water connections in their homes or yards.4 The rest—an estimated 2.2 billion persons—obtain water for their needs from shared point sources such as taps, boreholes, and wells. Even when such water points deliver relatively high-quality supply, they are located at some distance from users' dwellings, providing opportunities for recontamination of water supplies during transport and storage.5 Efforts to improve and maintain the quality of stored water in the home have included the development of many safe water storage and point of use water treatment technologies, several of which have been shown to be effective for reducing contamination by fecal indicator bacteria (FIB) in experimental settings.3,6,7
Along with sound household water management, hand hygiene is a second important health behavior associated with reducing respiratory and diarrheal illness. Handwashing with soap, for example, has been estimated to reduce the risk of diarrhea by 47%.8 Several randomized controlled trials have documented significant reductions in diarrheal and respiratory illness from handwashing in experimental settings.9,10 In theory, hand hygiene improvements could reduce both the transmission of pathogens through interpersonal contact as well as the risk that stored water and food will be contaminated through handling.
The experimental efficacy of these interventions notwithstanding, it has proven much more challenging to sustain water treatment, safe water storage, and hand hygiene behaviors within everyday settings. In one rural population of Guatemala, for example, researchers initially documented a 39% reduction in diarrhea after a field experiment involving a point of use flocculant disinfectant product.11 A second study carried out 6 months later in the same population found that only 5% of households had recently purchased the flocculant disinfectant, despite its wide availability and shown efficacy against diarrheal illness.12 In Pakistan, a follow-up study carried out 18 months after a successful handwashing intervention documented significantly better handwashing technique for some indicators among treatment versus control households; however, no significant difference in soap purchase or rates of diarrheal illness were observed.13
Given that the impacts of hand hygiene, water treatment, and water storage technologies on health are critically mediated by behavior, it is important to understand what types of interventions are effective in promoting correct and sustained use. Research into other behavior-mediated health technologies (e.g., seatbelts and condoms) has documented the importance of providing accurate information about the potential impacts of behavior change on health and other quality of life elements for both initial adoption as well as for longer-term maintenance of the behaviors under study.14,15 Moreover, it is well-understood that health messages that are relatively more tailored to the target population have greater impact on behavior change.16–18 In the water, sanitation, and hygiene field, messages regarding water management and hand hygiene have historically been untargeted in nature and are often delivered as a one-off exercise during the installation of a new water point.19
In previous research, Jalan and Somanathan20 tested the water supply of 965 urban households in India; they provided presence/absence results for the fecal indicator bacteria Escherichia coli to 497 treatment households and monitored the remaining 468 control households. After 8 weeks, households that had received a positive result were 11% more likely to report having made changes in water purification, handling, and/or storage behavior compared with those in the control group. No samples of water were analyzed after the intervention, and there was no effort made to compare the impact of household water testing with the kind of social marketing or educational programs that are more commonly used in developing countries. To our knowledge, no other published studies examine the effect of contamination information on household perceptions or activities.
This paper presents the results of a field study investigating the effects of providing households with individualized water and hand-rinse test E. coli results along with general health messages related to water, sanitation, and hygiene. Impacts of the intervention on knowledge of strategies to reduce risk of gastrointestinal and respiratory illness, practice of safe water management and hand hygiene, and microbial contamination of stored water and respondents' hands were assessed. Our objective was to determine whether the provision of test result information, along with health messages about water, hygiene, and health, leads to comparatively greater gains for each of these outcomes relative to providing health messaging alone.
Study Site and Experimental Methods
Dar es Salaam (6°48′S, 39°17′E) is the largest city in the east African country of Tanzania. With an estimated 2.8 million inhabitants in 2006, Dar es Salaam is the ninth fastest growing city on the planet, with a population doubling time of just 16.4 years.21
Sample frame.
Within the peri-urban zones of Dar es Salaam, three communities were selected for the study that had recently received upgrades in water supply infrastructure from the Community Water Supply and Sanitation Program (CWSSP) administered by the Dar es Salaam Water Supply and Sanitation Authority. The water supply infrastructure upgrades included installation of one or more deep borewells, an elevated storage tank, and a limited distribution network with public taps. Additional information about the CWSSP is presented in the Supplemental Information.
In the three communities selected for the study, a list of every 10 cell—administrative units of 10–30 housing units—was obtained from a community leader. From these, a random draw of 20 10-cell units was carried out for each community. Participatory mapping of each housing unit in the selected 10 cells was undertaken to identify those households that included children under the age of 5 years, because this population is the most vulnerable to water- and sanitation-related disease. A total of 350 households with under 5-year-old children was randomly selected from this parent population. Female heads of household were targeted for participation given their principal role in water management and health care in their families. Between 96 and 121 households agreed to participate in each of the three communities, for a total of 334 households. Full informed consent procedures were completed for each participant, and approval for the research was obtained by institutional review boards in the United States and Tanzania.
Data and sample collection.
The fieldwork was carried out over the period from June to September 2008, during the dry season. Each enrolled household was visited four times, approximately one time every 2 weeks. Attrition during the second, third, and fourth visits resulted in a final sample of 248 households with complete data. Analysis of household characteristics for participants who dropped out revealed only one significant difference: these families had fewer under 5-year-old children, on average, compared with the households that participated for the entire duration of the study. Fifty-one percent of the 86 mothers who left the study were traveling out of Dar es Salaam; 45% withdrew, and 4% were ill or lost the under 5-year-old child in their household. Additional information on attrition and sample composition is provided in Supplemental Figure S1.
Four in-depth survey instruments—baseline (BL), informational intervention (IN), and two follow-up visits (F1 and F2)—were developed, translated into Kiswahili, and programmed into a software package for loading onto personal digital assistants (PDAs). Each survey instrument included sections on socioeconomic and demographic characteristics of the household, water supply and sanitation services, family health, knowledge of water, sanitation, hygiene, and health linkages, and the respondent's self-efficacy. For all respondents at each household at each visit, local enumerators carried out the in-depth questionnaire with the female head of household and collected water and hand-rinse samples.
Water samples of 500 mL were collected by asking the respondent to retrieve water that would be used for drinking from the storage container in the manner typically used by household members (e.g., decanting or dipping a cup or ladle into the water). The respondent deposited the water into a sterile Whirl-Pak bag (NASCO Corp., Fort Atkinson, WI), which was sealed by the enumerator using sterile technique. Sodium thiosulfate was added to the water sample bag immediately before collection as a precaution to neutralize any chlorine present in the water. The enumerator also recorded the type of water source from which the water had been obtained (a CWSSP borewell, a neighbor with a household connection to the municipal network delivering chlorinated surface water, or a cart vendor). Hand-rinse samples were sought from each respondent (female head of household). Each participant inserted each of her hands into a 69-oz Whirl-Pak bag containing 350 mL sterile water. The subject agitated each hand in the water for 15 seconds; this was followed by an additional 15 seconds of massaging by the enumerator through the bag. Further details of this method can be found in Pickering and others.22
All water and hand-rinse samples were stored on ice in a cooler during transport back to the laboratory. Samples were processed within 4 hours of collection. Each sample was analyzed for concentrations of E. coli using US Environmental Protection Agency (USEPA) method 1604.23 For stored water samples, 100-mL samples were processed by membrane filtration, providing a lower and upper detection limit of 1 and 500 colony forming units (CFU)/100 mL, respectively. For hand-rinse samples, 10 mL were filtered, providing a lower detection limit of 35 CFU per two hands and an upper detection limit of 17,500 CFU per two hands. If no colony-forming units were visible on the plates, then a value of 0.5 CFU was assigned to the plate. If there were too many colony-forming units to count, then a value of 500 CFU (the upper detection limit) was assigned to the plate count. Bacterial counts were normalized such that stored water concentrations are expressed as CFU per 100 mL and hand-rinse concentrations are expressed as CFU per two hands.
Intervention.
Each participating household received one of four different informational treatments during the second visit by enumerators. One-quarter (the information cohort) received information about a variety of strategies that can be used to reduce risk of water- and sanitation-related illness, similar to the materials provided through the CWSSP. The costs and expected benefits of various strategies were discussed with each respondent. Respondents in the other three cohorts received this same information as well as their own water and/or hand-rinse test results (test cohorts). The second cohort, the water cohort, received water quality test results only; the third cohort, the hand cohort, received hand-rinse test results only. The fourth cohort, the water/hand cohort, received both types of test results. Members of all cohorts received all of their test results after the conclusion of the study. All households within a given 10 cell received the same cohort assignment (i.e., randomization was carried out at the 10-cell level).
Each test result was explained in language appropriate to the local context. A handout was provided to the respondent that contained photos of each type of sample taken (e.g., stored drinking water and hands). The handout included (1) the actual concentration of E. coli in the respondent's water and/or hand-rinse samples, the meaning of which was carefully explained by the enumerator, (2) for the water tests only, the median concentration of stored waters from similar sources (e.g., borewell, municipal piped water connection, or cart vendor) across all participating households in the respondent's community, and (3) ordinal categorizations of the household's results as low (0–10 CFU/100 mL [water test] or < 35 CFU/2 hands [hand test]), medium (11–100 CFU/100 mL [water test] or 35–100 CFU/2 hands [hand test]) or high (> 100 CFU/100 mL or > 100 CFU/2 hands). The classification scheme for water test results follows the World Health Organization guidelines24 for rural water. No published classification scheme for hand contamination currently exists, and therefore, we opted to define any hand test result lower than our detection limit (35 CFU/2 hands) as low and follow the same categorizations for the medium and high designations that were used for the water tests.
Water management and hand hygiene behaviors were assessed largely through self-report, although some visual confirmations were also carried out by enumerators (e.g., documenting whether water storage containers were covered). Respondents were asked at each visit about the frequency with which they engaged in hand hygiene and water management behaviors that could reduce risk of water- and sanitation-related illness. During follow-up visits, respondents were also asked open-ended questions about any behavioral changes their households had made during the period since the enumerators' last visit. Those reports not corroborated with spot checks or observation are vulnerable to social desirability effects and recall bias, which have been documented by other researchers.24–26
Statistical methods.
All bacterial concentrations were log10-transformed (referred to as log) before analysis. The t test and the χ2 test were used to test for differences in baseline characteristics between the information and test cohorts for continuous and categorical variables, respectively. Because baseline levels of E. coli contamination varied across cohorts, analysis of covariance (ANCOVA) was used to test for significant differences in changes of contamination levels between baseline and follow-up visits. Logistic and ordinary least squares regression analyses were used to test for significant associations between respondent and household characteristics, cohort assignment, test result content, and several dependent variables of interest. All statistical analyses were performed with SPSS Statistics 17.0 (SPSS Inc., Chicago, IL).
Results
Household characteristics.
The typical family in our sample is comprised of five persons. Forty-three percent of study households live in a single-family home, whereas another 51% share a housing unit with one to six other families. More than 90% of housing units have concrete walls and floors and tin roofs. One-half of study households have in-home electricity service, and 17% have a shallow well (typically 2–10 m in depth) on their plot. All households in the study have access to a private sanitation facility (either exclusive or shared with other households). All are on-site facilities, 52% of which have reinforced pits or septic tanks, whereas 48% have simple unlined pits.
Apart from collecting rainwater during the rainy season, most households use one or two principal water sources. Approximately 80% of households obtain drinking water from the CWSSP borewells, another 10% get water from neighbors with private connections to the municipal network, 9% get water from vendors who deliver water from a variety of sources to the home, and 1% get water from other sources such as shallow wells and commercially sold bottled water. The sources of study households' drinking water exhibited very little change over the course of the study.
Overall, respondents and households shared similar characteristics across the four cohorts. One exception includes the religious profile of the groups; the information cohort had a significantly lower share (39%) of respondents self-identifying as Muslim compared with the water cohort (58%; t = −2.43, degrees of freedom (df) = 167, P = 0.02). No other test for comparability between the information and test cohorts had a result with a P value less than 0.10. (See Supplemental Table S1 for additional details on cohort characteristics.)
Changes in knowledge.
During the baseline survey, respondents were asked whether each of four known causes of gastrointestinal illness (drinking contaminated water, eating spoiled food, having poor personal hygiene, and having contact with standing water in the environment) as well as two unsubstantiated factors (bad odors and bad spirits) could cause diarrheal illness in children. Respondents provided correct responses to an average of 74% of these prompts; no significant differences in knowledge of causes of diarrheal illness across cohorts at baseline were apparent (all t test P values > 0.40). These same questions were asked of respondents during both follow-up visits. The mean percentage of correct responses increased slightly to 76% during the first and second follow-up visits for the entire sample of respondents (both P > 0.10). No significant differences in mean changes of knowledge scores were observed between any test cohort and the information cohort (P ≥ 0.30 for all t tests). Similar patterns in responses regarding the causes of respiratory illness were observed (data not shown).
We also asked all respondents about their knowledge of water treatment methods. At baseline, 79% of respondents could name at least one method of making water used for drinking and cooking safer without prompting. The mean number of treatment methods cited among these respondents was 1.2 (median = 1). Boiling was the most frequently mentioned water treatment strategy, cited by 69% of respondents. Chlorination was named by 32%, and filtering water through a cloth was named by 17%. No significant differences in the number of water treatment methods known at baseline were observed between the information and the test cohorts (all P > 0.15).
Respondents were asked to name water treatment methods during both follow-up visits as well. Across the entire study sample, the mean number of methods doubled to 2.5 during the first follow-up visit (t = 17.6, df = 246, P < 0.001) and stayed at this value for the second follow-up visit (t = 14.4, df = 227, P < 0.001). These gains were generally distributed equally across the cohorts, as evidenced by the absence of significant differences in the change in mean number of treatment methods cited between each of the test cohorts and the information cohort (Table 1).
Table 1.
Baseline levels, changes in knowledge, reported water management, and hand hygiene practices by cohort
| Full sample (N = 334) | Information cohort (N = 79) | Hand cohort (N = 84) | Water cohort (N = 90) | Water/hand cohort (N = 81) | |
|---|---|---|---|---|---|
| Mean proportion causes of diarrhea correctly identified at baseline | 0.74 (0.16) | 0.73 (0.19) | 0.74 (0.16) | 0.75 (0.14) | 0.74 (0.13) |
| Mean change in proportion causes of diarrhea correctly identified BL to F1 | 0.02 (0.21) | 0.05 (0.23) | 0.02 (0.19) | −0.02 (0.23) | 0.03 (0.18) |
| Mean change in proportion causes of diarrhea correctly identified BL to F2 | 0.02 (0.21) | 0.03 (0.23) | 0.05 (0.20) | 0.03 (0.21) | −0.10 (0.18) |
| Mean number of water treatment methods cited at baseline | 1.21 (0.81) | 1.29 (0.88) | 1.12 (0.73) | 1.24 (0.81) | 1.20 (0.80) |
| Mean change in number of water treatment methods cited BL to F1 | 1.32* (1.16) | 1.20 (1.26) | 1.57 (1.06) | 1.29 (1.23) | 1.26 (1.03) |
| Mean change in number of water treatment methods cited BL to F2 | 1.25* (1.30) | 1.17 (1.38) | 1.42 (1.16) | 1.28 (1.39) | 1.16 (1.22) |
| Mean daily reported handwashing rates (times per day) at baseline | 3.78 (1.35) | 3.48 (1.46) | 3.91 (1.30) | 3.91 (1.36) | 3.77 (1.28) |
| Change in reported mean daily handwashing rates BL to F1 | 0.48* (1.38) | 0.50 (1.71) | 0.42 (1.41) | 0.52 (1.30) | 0.46 (1.17) |
| Change in reported mean daily handwashing rates BL to F2 | 0.69* (1.50) | 1.20 (1.77) | 0.66 (1.43) | 0.52† (1.28) | 0.52† (1.50) |
| Percentage reporting more soap use since BL at F1 | 0.39 (0.49) | 0.27 (0.44) | 0.37 (0.49) | 0.46‡ (0.50) | 0.44‡ (0.50) |
| Percentage reporting treatment of drinking water at baseline | 0.56 (0.50) | 0.57 (0.50) | 0.46 (0.50) | 0.66 (0.48) | 0.55 (0.50) |
| Change in percentage reporting treatment of drinking water BL to F1 | 0.11* (0.45) | 0.22 (0.42) | 0.13 (0.48) | 0.00‡ (0.47) | 0.14 (0.40) |
| Change in percentage reporting treatment of drinking water BL to F2 | 0.11* (0.55) | 0.21 (0.56) | 0.31 (0.60) | 0.00 (0.55) | 0.00‡ (0.45) |
| Percentage reporting improved water storage since BL at F1 | 0.31 (0.46) | 0.31 (0.46) | 0.24 (0.43) | 0.34 (0.48) | 0.36 (0.48) |
Standard deviations in parentheses.
Change for full sample is significantly different from zero (P < 0.05).
Difference between follow-up 2 and baseline is significantly different from information cohort (P < 0.05).
Difference between follow-up 1 and baseline is significantly different from information cohort (P < 0.05).
Changes in hand hygiene behavior.
At each household visit, mothers were asked how many times they had washed their hands the day before the visit. At baseline, respondents reported a mean of 3.5 (information cohort) to 3.9 (hand and water cohorts) instances of handwashing in the day before the interview. No significant differences in reported rates of handwashing were observed between the test cohorts and the information cohort at baseline (all P > 0.10).
For the entire set of study participants, mean reported handwashing instances in the day before the interview increased from 3.8 to 4.3 (t = 4.78, df = 178, P < 0.001) between baseline and the first follow-up visit. The mean handwashing rate increased further to 4.5 instances per day across all respondents at the second follow-up visit (t = 6.13, df = 176, P < 0.001). Members of the information cohort had significantly greater increases in reported handwashing between the baseline and second follow-up visit compared with both the water cohort (t = 2.1, df = 68, P = 0.04) and the water/hand cohort (t = 2.1, df = 82, P = 0.04). No significant differences between the information and hand cohorts were observed in reported handwashing behaviors, and there were no significant differences observed between the information cohort and any test cohort at the first follow-up visit.
During follow-up visits, respondents were also asked an open-ended question about whether their household had made any changes in their hand hygiene practices. No significant differences were observed between the information cohort and each of the test cohorts with regard to the percentage of respondents that mentioned increased handwashing at critical times (e.g., before food preparation and after defecation) or increased handwashing of children (all P > 0.20). A higher percentage of mothers in the water (46%) and water/hand cohort (44%) compared with the information cohort (27%) mentioned more frequent use of soap for handwashing (both P < 0.02).
Changes in water management practices.
At baseline, reported water treatment practices were fairly consistent across the cohort. Between 36% and 47% of respondents in each cohort reported always or almost always boiling the water used for drinking and cooking in the household. Between 9% and 14% of respondents in each cohort reported regularly filtering water by pouring it through a cloth stretched over the storage container. Between 7% and 13% reported using a chlorine product to disinfect drinking water. No significant differences were observed between the share of respondents reportedly treating their family's drinking water in the information versus any of the test cohorts at baseline (all P > 0.20).
Between the baseline and first follow-up visit, the share of all respondents who said that they were treating their drinking water increased from 56% to 68% (t = 3.8, df = 231, P < 0.01). This percentage fell slightly to 66% at the second follow-up visit but was still significantly higher than baseline (t = 2.8, df = 201, P < 0.01). The change in the percentage of respondents who said they were regularly treating their household's drinking water between the baseline and second follow-up was significantly greater for the information cohort (an increase of 21% points) compared with the water cohort (no change; t = 2.7, df = 110, P < 0.01). Similarly, the change in percentage of mothers in the information cohort who said they were regularly treating their household's drinking water was greater for the information cohort than for the water/hand cohort (no change; t = 2.0, df = 81, P = 0.05). No significant difference in rates of reported water treatment was observed between the information and hand cohorts.
During the follow-up visits, respondents were also posed several open-ended questions about any changes that their household had made in water storage practices. Roughly 30% of respondents in each cohort said that they were making efforts to keep stored water covered more consistently, and 25% said that they had tried to reduce the frequency with which families members dipped their hands into storage containers. No significant differences in reported changes of water storage practices were observed between members of the information cohort and each of the test cohorts (all P > 0.15).
Multivariate modeling of reported changes in water management and hand hygiene practices.
Multivariate regression analyses were carried out to explore the factors associated with changes in reported water storage, water treatment, and hand hygiene behaviors recorded during the first follow-up visit (Table 2). Overall, the explanatory power of these models is low, suggesting that important variables have been omitted. Two models of reported changes in drinking water treatment and handwashing with soap were fit to evaluate differences in baseline conditions across study participants. The first model includes all study participants along with a dummy variable representing baseline status for the relevant behavior (e.g., regular water treatment and frequency of handwashing with soap). The second model is restricted only to study participants whose baseline performance of the relevant behavior was poor (and thus, arguably had greater scope for improvement after the intervention). Specifically, only data collected from mothers who said at baseline that their households did not treat their drinking water and those who did not have soap in their homes at the baseline interview were used for Models 2B and 3B, respectively. (A second model of water storage practices could not be fit, because baseline water storage practices evaluated in the study exhibited little variation across participants.)
Table 2.
Logistic regression of reported behavior changes between baseline and first follow-up survey
| Mean (SD) value for full sample | Reported improved water storage practices | Reported initiation or increased frequency of drinking water treatment | Reported initiation of or more frequent handwashing with soap | |||
|---|---|---|---|---|---|---|
| Model 1: full sample, N = 328 (SE) | Model 2A: full sample, N = 332 (SE) | Model 2B: no reported water treatment at baseline, N = 136 (SE) | Model 3A: full sample, N = 332 (SE) | Model 3B: no soap observed at baseline, N = 91 (SE) | ||
| Intercept | – | −0.83 (0.25) | −1.45 (0.34) | −1.00 (0.45) | −1.03 (0.31) | −1.30 (0.53) |
| Hand cohort (dummy) | 0.25 (0.44) | −0.33 (0.35) | −0.45 (0.44) | −0.70 (0.62) | 0.48 (0.34) | 0.45 (0.70) |
| Water cohort (dummy) | 0.27 (0.44) | 0.02 (0.35) | 0.28 (0.41) | −0.20 (0.66) | 0.78* (0.35) | 1.31* (0.68) |
| Hand/water cohort (dummy) | 0.24 (0.43) | 0.12 (0.35) | 0.25 (0.42) | −0.72 (0.69) | 0.74* (0.35) | 1.39* (0.71) |
| High water test result received (dummy) | 0.15 (0.36) | 0.52 (0.35) | −0.37 (0.43) | 0.37 (0.70) | 0.09 (0.34) | 0.17 (0.67) |
| Beyond primary education (dummy) | 0.23 (0.42) | – | – | −1.07 (0.79) | – | −0.38 (0.66) |
| Reported drinking water treatment at BL (dummy) | 0.56 (0.50) | – | 0.14 (0.29) | – | – | – |
| Reported regular soap use at BL (dummy) | 0.42 (0.49) | – | – | – | 0.02 (0.26) | – |
| −2 log likelihood | 406.81 | 305.73 | 125.21 | 434.15 | 112.09 | |
| Quasi R2 | 0.08 | 0.03 | 0.05 | 0.03 | 0.12 | |
SD = standard deviation; SE = standard error.
0.01 < P ≤ 0.05.
With all other variables held constant, cohort membership was significantly associated with reported increases in soap for handwashing. Members of the water and hand/water cohort were more likely to report having increased soap use after the intervention compared with those in the information cohort (all P < 0.05). In Model 3B, the average cohort effect is particularly large, with water and hand/water cohort members being 3.7 and 4.0 times, respectively, more likely than those in the information cohort to report increased use of soap for handwashing. Receiving a high stored water test result (as opposed to simply receiving test results or not) was not significantly associated with the reported behavior changes modeled. The association between hand-rinse test content and reported behavior could not be evaluated because of the much lower variation in hand test results. Among the mothers who received a hand-rinse test result (all members of the hand or water/hand cohorts), 85% had a high result. The high degree of collinearity with cohort assignment makes it infeasible to uniquely assess the contribution of test result content to reported changes in hand hygiene behavior.
Changes in bacterial contamination of stored water and mothers' hands.
At baseline, contamination of respondents' stored drinking water with E. coli ranged between 1.3 and 1.7 log CFU/100 mL across the four cohorts. The mean contamination in untreated versus reportedly treated stored water was 1.45 and 1.40 log CFU/100 mL, respectively. Because these contamination levels were not significantly different (t = 0.4, df = 326, P = 0.68), treated and untreated waters were considered in aggregate for all analyses.
Across all study participants, contamination of stored drinking water was higher by an average of 0.12 log CFU/100 mL between baseline and the first follow-up visit and higher by an average of 0.09 log CFU/100 mL between baseline and the second follow-up visit (P > 0.20 for both comparisons). To compare estimated marginal mean changes in the log-transformed contamination levels across the cohort given the differing baseline contamination levels, one-way analysis of covariance was used (Figure 1). No significant differences were detected between the information and each of the test result cohorts with respect to the change in the estimated marginal mean level of stored drinking water E. coli contamination between baseline and either the first or second follow-up visits (all P values > 0.20).
Figure 1.
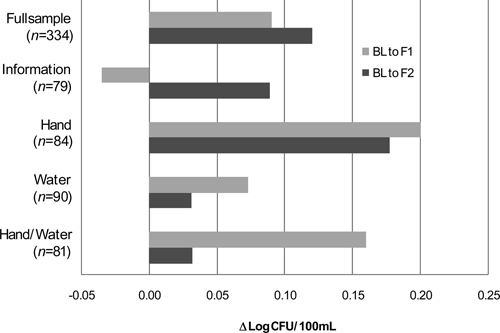
Change in estimated marginal mean concentration log-transformed E. coli (log CFU/100 mL) in stored drinking water by cohort and study phase. Negative values indicate a reduction in contamination.
Contamination of mothers' hands with E. coli during the baseline visit ranged between 2.9 and 3.4 log CFU/2 hands across the four cohorts. Across all study participants, the average concentration of E. coli on mothers' hands was 0.32 log CFU/2 hands lower in the first follow-up compared with the baseline (t = 4.06, df = 241, P < 0.001). No significant difference was detected in the mean level of hand contamination between the second follow-up and the baseline (t = 0.82, df = 198, P = 0.42).
One-way analysis of covariance was used to adjust cross-cohort comparisons for baseline differences in hand contamination. No significant differences between the information and the hand cohort were detected when comparing estimated marginal mean changes in contamination between baseline and either follow-up visit (Figure 2). The mean estimated marginal change between baseline and the second follow-up for the information cohort—a reduction of 0.29 log CFU/2 hands—was 0.36 log CFU greater than that of the water cohort (P = 0.04) and 0.37 log CFU greater than that of the water/hand cohort (P = 0.05).
Figure 2.
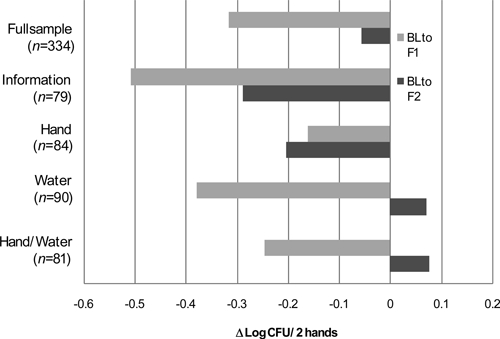
Change in estimated marginal mean concentration of log-transformed E. coli (log CFU/2 hands) on mothers' hands by cohort and study phase. Negative values indicate a reduction in contamination.
Multivariate modeling of changes in water and hand contamination.
Multivariate regression analyses were used to explore the factors associated with changes in log-transformed E. coli contamination of stored drinking water and mothers' hands. Table 3 presents model results for the changes observed between baseline and the first follow-up visit (results obtained for the second follow-up visit are presented in the Supplemental Information). On average, members of the hand cohort exhibited a 0.40 log CFU/2 hands increase in hand contamination during the follow-up visit compared with the information cohort (P < 0.05) after controlling for baseline conditions and reported behavior change after the intervention. No other cohort effect was observed. Additionally, receipt of a high water test result was not significantly associated with mean changes in E. coli levels of stored drinking water (P > 0.20).
Table 3.
Ordinary least-squares regression of change in log mean E. coli contamination of stored water and hands between baseline and first follow-up survey
| Mean (SD) value for full sample | Model 1: change in stored water contamination (log CFU/100 mL) | Model 2: change in hand contamination (log CFU/2 hands) | |
|---|---|---|---|
| Intercept | – | 1.59 (0.22) | 2.22 (0.29) |
| Member of hand cohort (dummy) | 0.24 (0.43) | 0.17 (0.20) | 0.40* (0.14) |
| Member of water cohort (dummy) | 0.31 (0.46) | −0.08 (0.20) | 0.17 (0.18) |
| Member of hand/water cohort (dummy) | 0.22 (0.42) | −0.03 (0.21) | 0.30 (0.20) |
| Received high water test result (dummy) | 0.15 (0.36) | 0.30 (0.24) | – |
| Baseline E. coli level (log CFU) | Water: 1.36 (0.98); hand: 3.20 (0.93) | −1.04† (0.08) | −0.81† (0.07) |
| Reported improved water storage (dummy) | 0.26 (0.44) | 0.05 (0.15) | – |
| Reported increased water treatment (dummy) | 0.26 (0.44) | −0.11 (0.16) | – |
| Reported increased handwashing frequency (dummy) | 0.24 (0.42) | −0.27‡ (0.16) | −0.14 (0.16) |
| Reported increased use of soap (dummy) | 0.47 (0.50) | 0.09 (0.14) | −0.30* (0.13) |
| Completed education beyond primary level (dummy) | 0.22 (0.42) | −0.41* (0.16) | 0.05 (0.16) |
| Adjusted R2 | – | 0.49 | 0.37 |
| N | – | 238 | 240 |
Negative coefficients indicate reduction in mean contamination. SD = standard deviation.
P ≤ 0.01.
0.01 < P ≤ 0.05.
0.05 < P ≤ 0.10.
Variations in baseline levels of contamination account for most of the changes observed at the follow-up visits, consistent with the notion that repeated measures of non-random subsamples from a population will trend to the mean value for the entire sample.27 At the same time, mothers who reported that their families had increased their use of soap for handwashing after the informational intervention had a reduction of 0.3 log CFU/2 hands more E. coli than those reporting no increase in soap usage, with all other variables held constant (P = 0.02). However, this effect did not persist to the second follow-up visit. Interestingly, the mean contamination of stored drinking water was 0.27 log CFU/100 mL lower in households where mothers reported an increase in the frequency of handwashing in the post-intervention period. The effect was only marginally significant (P = 0.09) but did persist into the second follow-up, with the mean difference in stored water contamination increasing to 0.36 log CFU/100 mL compared with baseline (P = 0.09). Finally, mothers who had completed formal education beyond primary school had an average of 0.41 log CFU/100 mL greater reduction of drinking water contamination compared with those with less education. This association was no longer evident at the second follow-up visit.
Test cohort participants' reaction to intervention.
In general, test cohort members were eager to receive their test results and discuss them with enumerators. Among mothers who received stored water test results during the intervention visit, 89% reported discussing those results within their families, and 39% said they discussed their result with persons outside the family, most often a neighbor. A greater percentage of mothers who received low water test results shared this information with others compared with mothers who received medium or high results, but the difference was not statistically significant (P = 0.13). Fifty-one percent of mothers who received a water test result said that it showed their family's drinking water to be more contaminated than they expected, whereas 43% said that the results were either consistent with their expectations (33%) or indicated lower levels of contamination than they had expected (10%). Reactions to the hand-rinse test results were very similar; 87% of mothers reported discussing the result with family members, and 34% discussed it with others outside the family. Fifty-four percent of mothers who received a hand-rinse test result said that it showed their hands to be more contaminated than they expected, whereas 40% said that the results were either consistent with their expectations (31%) or indicated lower levels of contamination than they had expected (9%).
Discussion
This study assessed the feasibility and utility of using stored water and hand-rinse test results as informational interventions to motivate improved water management and hand hygiene in a resource-constrained setting. Across all cohorts, study participants significantly increased the number of water treatment methods that they could name without prompting, rates of reported handwashing, and rates of reported drinking water treatment between the baseline and follow-up visits. A substantial proportion of mothers also said that their families made efforts to improve their water management and hand hygiene practices after the informational intervention. The potential for social desirability and repeated testing effects to generate upward bias in these self-reported data should be borne in mind.
Indeed, evidence regarding the translation of these reported behavioral changes and knowledge gains into lower rates of FIB contamination in the household environment is mixed. Across all cohorts, mothers had an average of 26% and 19% lower levels of E. coli contamination on their hands at their first and second follow-up visits, respectively, compared with baseline levels (both P < 0.01). By contrast, mean levels of E. coli found in stored drinking water increased by 29% and 20% between baseline and follow-up 1 and 2, respectively, although these changes were not statistically significant (both P > 0.20).
The short duration of the study limits our ability to draw conclusions about the persistence of these changes in households' drinking water management and hand hygiene. Data collected during the second follow-up visit, however, are suggestive of diminishing effects less than 2 mo after the intervention. It should also be noted that, because this study did not include a true control group (i.e., households from whom information and samples were obtained without the provision of either health information or test results), the impact of the household visit itself cannot be distinguished from that of the health message intervention.
Given the limited duration of this study, the impact of the interventions on health outcomes was not a focus of the research. Incidence of respiratory and gastrointestinal symptoms was tracked, however, to define incidence of respiratory illness (RI), highly credible RI (HCRI), gastrointestinal illness (GI), and highly credible GI (HCGI). No statistically significant change in rates of illness was observed for the full sample of mothers across study phases, and rates of change were not significantly different for the information versus each of the test cohorts (all P > 0.20). Additional work is needed to determine whether the hand hygiene and water management behavior changes observed among study participants had any impact on the health of their household members.
These caveats acknowledged, evidence from the multivariate analysis suggests that water and hand/water test cohort members had significantly higher rates of self-reported behavior change compared with those in the information cohort. At the same time, information cohort members exhibited a significantly greater reduction of E. coli on their hands compared with the test cohorts. No significant change in mean E. coli levels of study participants' stored drinking water was observed for any cohort. Taken together, these findings suggest that respondents who received household test results in this study were more likely to report behavioral improvements compared with the information cohort but were equally or less likely to experience actual reductions in E. coli contamination in water and on hands, respectively. The cost and logistical demands of incorporating bacteriological testing into campaigns to improve household water management and hand hygiene can be considerable. The evidence from this study suggests that additional research is needed to elucidate conditions under which such testing can deliver cost-effective results.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Woods Institute for the Environment and the Presidential Fund for Innovation in International Studies, both of Stanford University. The authors thank Cynthia Castro, Fred George, Prosper Chaki, the staff of the Health and Environmental Rescue Organization, the members of our field and laboratory teams, and participating households for their assistance with this research. The authors thank Maggie Montgomery and Tim Julian for helpful input on the manuscript. The authors are also grateful to two anonymous reviewers who provided thoughtful and thorough feedback on an earlier draft.
Note: Supplemental data appear at www.ajtmh.org.
Footnotes
Authors' addresses: Jennifer Davis, Department of Civil and Environmental Engineering and Woods Institute for the Environment, Stanford University, Stanford, CA, E-mail: jennadavis@stanford.edu. Amy J. Pickering, Emmett Interdisciplinary Program in Environment and Resources, Stanford University, Stanford, CA, E-mail: amyjanel@stanford.edu. Kirsten Rogers, Human Biology Program, Stanford University, Stanford, CA, E-mail: kirstenr@stanford.edu. Simon Mamuya, Department of Environmental and Occupational Health, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania, E-mail: smamuya@muhas.ac.tz. Alexandria B. Boehm, Department of Civil and Environmental Engineering, Stanford University, Stanford, CA, E-mail: aboehm@stanford.edu.
References
- 1.Cutler D, Miller G. The role of public health improvements in health advance: the twentieth-century United States. Demography. 2005;42:1–22. doi: 10.1353/dem.2005.0002. [DOI] [PubMed] [Google Scholar]
- 2.Esrey SA, Feachem RG, Hughes JM. Interventions for the control of diarrhoeal diseases among young children: improving water supplies and excreta disposal facilities. Bull World Health Organ. 1985;63:757–772. [PMC free article] [PubMed] [Google Scholar]
- 3.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 4.Joint Monitoring Programme . Progress on Drinking Water and Sanitation: Special Focus on Sanitation. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 5.Wright J, Gundry S, Conroy J. Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Trop Med Int Health. 2004;9:106–117. doi: 10.1046/j.1365-3156.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- 6.Arnold BF, Colford JM., Jr Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: a systematic review and meta-analysis. Am J Trop Med Hyg. 2007;76:354–364. [PubMed] [Google Scholar]
- 7.Clasen T, Roberts I, Rabie T, Schmidt W, Cairncross S. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev. 2006;3:CD004794. doi: 10.1002/14651858.CD004794.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis. 2003;3:275–281. doi: 10.1016/s1473-3099(03)00606-6. [DOI] [PubMed] [Google Scholar]
- 9.Luby SP, Agboatwalla M, Raza A, Sobel J, Mintz ED, Baier K, Hoekstra RM, Rahbar MH, Hassan R, Qureshi SM. Microbiologic effectiveness of hand washing with soap in an urban squatter settlement, Karachi, Pakistan. Epidemiol Infect. 2001;127:237–244. doi: 10.1017/s0950268801005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. Am J Public Health. 2008;98:1372–1381. doi: 10.2105/AJPH.2007.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiller TM, Mendoza CE, Lopez MB, Alvarez M, Hoekstra RM, Keswick BH, Luby SP. Reducing diarrhoea in Guatemalan children: randomized controlled trial of flocculant-disinfectant for drinking-water. Bull World Health Organ. 2006;84:28–35. doi: 10.2471/blt.04.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luby SP, Agboatwalla M, Bowen A, Kenah E, Sharker Y, Hoekstra RM. Difficulties in bringing point-of-use water treatment to scale in rural Guatemala. Am J Trop Med Hyg. 2008;78:382–387. [PubMed] [Google Scholar]
- 13.Luby SP, Agboatwalla M, Bowen A, Kenah E, Sharker Y, Hoekstra RM. Difficulties in maintaining improved handwashing behavior, Karachi, Pakistan. Am J Trop Med Hyg. 2009;81:140–145. [PubMed] [Google Scholar]
- 14.Bandura A. The primacy of self-regulation in health promotion. Appl Psychol. 2005;54:245–254. [Google Scholar]
- 15.Maes S, Karoly P. Self-regulation assessment and intervention in physical health and illness: a review. Appl Psychol. 2005;54:267–299. [Google Scholar]
- 16.Abrams DB, Mills S, Bulger D. Challenges and future directions for tailored communication research. Ann Behav Med. 1999;21:299–306. doi: 10.1007/BF02895961. [DOI] [PubMed] [Google Scholar]
- 17.Strecher V, Wang C, Derry H, Wildenhaus K, Johnson C. Tailored interventions for multiple risk behaviors. Health Educ Res. 2002;17:619–626. doi: 10.1093/her/17.5.619. [DOI] [PubMed] [Google Scholar]
- 18.Smeets T, Kremers SP, de Vries H, Brug J. Effects of tailored feedback on multiple health behaviors. Ann Behav Med. 2007;33:117–123. doi: 10.1007/BF02879892. [DOI] [PubMed] [Google Scholar]
- 19.Iyer P, Davis J, Yavuz E, Evans B. Rural Water Supply, Sanitation, and Hygiene: A Review of 25 Years of World Bank Lending (1978–2003) Washington, DC: World Bank; 2005. [Google Scholar]
- 20.Jalan J, Somanathan E. The importance of being informed: experimental evidence on demand for environmental quality. J Dev Econ. 2008;87:14–28. [Google Scholar]
- 21.City Mayors The World's Fastest Growing Cities and Urban Areas from 2006 to 2020. 2009. http://www.citymayors.com/statistics/urban_growth1.html Available at. Accessed May 17, 2009.
- 22.Pickering AJ, Davis J, Walters SP, Horak HM, Keymer DP, Mushi D, Strickfaden R, Chynoweth JS, Liu J, Blum A, Rogers K, Boehm AB. Hands, water, and health: fecal contamination in Tanzanian communities with improved, non-networked water supplies. Environ Sci Technol. 2010;44:3267–3272. doi: 10.1021/es903524m. [DOI] [PubMed] [Google Scholar]
- 23.United States Environmental Protection Agency . Method 1604: Total Coliforms and Escherichia coli in Water by Membrane Filtration Using a Simultaneous Detection Technique (MI Medium) United States Environmental Protection Agency; 2002. [Google Scholar]
- 24.World Health Organization . Water Quality Guidelines for Rural Drinking Water Supplies. Surveillance and Control of Community Supplies. 2nd ed. Geneva, Switzerland: World Health Organization Press; 1997. [Google Scholar]
- 25.Stanton B, Clemens J. Twenty-four hour recall, knowledge-attitude-practice questionnaires, and direct observations of sanitary practices: a comparative study. Bull World Health Organ. 1987;65:217–222. [PMC free article] [PubMed] [Google Scholar]
- 26.Curtis V, Cousens S, Mertens T, Traoré E, Kanki B, Diallo I. Structured observations of hygiene behaviours in Burkina Faso: validity, variability, and utility. Bull World Health Organ. 1993;71:23–32. [PMC free article] [PubMed] [Google Scholar]
- 27.Davis C. The effect of regression to the mean in epidemiologic and clinical studies. Am J Epidemiol. 1976;104:493–498. doi: 10.1093/oxfordjournals.aje.a112321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


