Abstract
A recurrent focus of Rhipicephalus sanguineus infestation was investigated in a suburban area of southern California after reports of suspected Rocky Mountain spotted fever in two dogs on the same property. Abundant quantities of Rh. sanguineus were collected on the property and repeatedly from each dog, and Rickettsia massiliae DNA was detected by polymerase chain reaction (PCR). Whole blood and serum samples from four dogs were tested by using PCR and microimmunofluorescent assay for antibodies against spotted fever group rickettsiae. Serum samples from all four dogs contained antibodies reactive with R. massiliae, R. rhipicephali, R. rickettsii, and 364D Rickettsia but no rickettsial DNA was detected by PCR of blood samples. Serum cross-absorption and Western blot assays implicated R. massiliae as the most likely spotted fever group rickettsiae responsible for seropositivity. To our knowledge, this is the first detection of R. massiliae in ticks in California.
Introduction
Rickettsia massiliae Mtu1 was first isolated in 1990 from a Rhipicephalus turanicus tick collected from a horse in Le Sambuc, Bouches-du Rhone, France.1 Its formal taxonomic species description appeared in 1993.2 Other isolates of R. massiliae were later obtained from Rhipicephalus sanguineus from Spain (Bar29), France (Mtu5), Greece (GS), and Arizona (AZT80), USA.1,3–5 Different genotypes of R. massiliae have been detected in several other ticks of the so-called Rhipicephalus spp. complex including Mtu5 genotype in Rh. senegalensis, and Mtu1 genotype in Rh. sulcatus, Rh. lunulatus and Rh. mushamae in central Africa,6 and Bar29 genotype in Rh. sanguineus and Rh. turanicus in Switzerland.7 The distribution of R. massiliae and its association with different Rhipicephalus spp. was subsequently extended to other Mediterranean and African countries,8–17 and to Argentina.18 The reported prevalence of R. massiliae in ticks has varied from 4.7% to 18%.11,12,15 However, the true prevalence and range of distribution of R. massiliae in Rhipicephalus ticks are unknown, especially in the New World. Whether the specific genetic types of R. massiliae have adapted preferentially to different tick vectors, animal hosts, and whether their potential to cause febrile illness varies are unknown.
The first confirmed human case of R. massiliae infection was reported in 2006.19 It was diagnosed by polymerase chain reaction (PCR) and sequence characterization of a 1984 cell culture isolate from a blood sample collected from a man hospitalized in Sicily with fever, maculopapular rash on the palms and soles, mild hepatomegaly, and an eschar. In 2008 in France, a second patient was diagnosed with similar clinical symptoms that included two eschars on the thigh and buttock, and bilateral chorioretinitis with acute blindness.20 The latest case was diagnosed in Spain by retrospective PCR testing of eschar tissue from a female patient from Buenos Aires, Argentina.21
There may have been additional cases of human infection with R. massiliae. Children diagnosed with Mediterranean spotted fever in Catalonia, an area that is endemic for the Bar29 isolate of R. massiliae, were refractory to treatment with rifampin, tested as an alternative antibiotic when tetracycline is contraindicated.22,23 Rickettsia massiliae is resistant to rifampin in vitro, and R. conorii (the etiologic agent of Mediterranean spotted fever) is not resistant.4,5,23
The similar clinical manifestations, common vector within the area endemic for Mediterranean spotted fever, and primary use of cross-reactive serologic assays for diagnosis of rickettsial diseases complicate understanding the true prevalence and distribution of R. massiliae and associated human or potentially canine infections. A limited canine serosurvey in northeastern Spain demonstrated that 8.6–25% (n = 93) of Catalonian dogs were exposed to R. massiliae Bar29 and 4–20% to R. conorii or another cross-reacting Rickettsia.24 A survey of Rh. sanguineus-infested dogs in Seville Province, Spain, showed that 18% were carrying ticks infected with R. massiliae.12 Because dogs are recognized sentinels for human rickettsial diseases,25–27 these findings may correlate with a higher prevalence of human cases caused by R. massiliae in areas where this agent is found.
We report the results of an investigation triggered by reports of sick dogs living on a property in Los Angeles County, California, with sustained infestation by Rh. sanguineus. We found that R. massiliae is present in California ticks and show the efficient long-term maintenance of R. massiliae in this naturally infected population of Rh. sanguineus. We also report serologic evidence for canine exposure to this Rickettsia, and demonstrate the lack of obvious negative effect of canine anti-rickettsial antibodies on tick feeding and circulation of R. massiliae in those ticks.
Materials and Methods
Study description.
In August 2007 and March 2008, the Los Angeles County Veterinary Public Health and Rabies Control Program (LACVPH) was notified about two ill dogs on the same property suspected of having Rocky Mountain spotted fever, a rare condition in the area. Both dogs were seropositive to spotted fever group rickettsiae (SFGR), as established by testing at the commercial laboratory, and had a heavy infestation with the brown dog tick Rh. sanguineus. The LACVPH reviewed the clinical history, treatment outcome, and commercial laboratory testing results of these dogs. Whole blood treated with EDTA and serum were collected from both case dogs and from two housemate apparently healthy dogs and submitted to the Rickettsial Zoonoses Branch, Centers for Disease Control and Prevention (CDC) (Atlanta, GA) for further testing. Ticks were also collected from the property and dogs and submitted to the CDC for testing. The owner was advised on tick control and prevention measures.
Preparation of antigens for electrophoresis and Western blotting.
Rickettsia rickettsii strain Bitterroot, R. massiliae strain AZT80, R. rhipicephali strains 3-7-♀-6 and CA871, and 364D Rickettsia were cultivated in African green monkey kidney cells (E6-Vero), harvested, and purified from the host cells using centrifugation through 25–45% (w/v) Renografin (Nycomed, Inc., Princeton, NJ) gradients as described.28 Purified rickettsiae were kept frozen in aliquots (10 mg of protein/mL) in sterile distilled water at –80°C until used for electrophoresis and Western blotting.
For electrophoresis, suspensions of purified rickettsiae were diluted in water to a concentration of 3 mg of protein/mL, and an equal volume of 2× Laemmli sample solubilizing buffer (125 mM Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate, 20% glycerol, 10% β-mercaptoethanol, 0.001% bromophenol blue dye)29 was added at room temperature.
Indirect microimmunofluorescence testing.
Antigens consisted of the Rickettsia-infected nuclear fraction collected as a by-product of the rickettsial purification from infected cells; it was resuspended and frozen in SRM buffer (0.281 M sucrose, 5 mM potassium glutamate buffer, pH 7.0, supplemented with 1% Renografin-76 and 5 mM MgCl2), and gamma irradiated with 1,000,000 Rads. The stock was thawed, the SRM was removed after centrifugation, the pellet was suspended in phosphate-buffered saline, pH 7.2, and 10-μL aliquots of antigens were spotted onto glass slides, air-dried, and fixed in acetone.
Serum samples were screened by indirect immunofluorescent antibody (IFA) assay at consecutive 2-fold serum dilutions starting at 1:32 in a dilution buffer (phosphate-buffered saline, pH 7.4, supplemented with 1% bovine serum albumin and 1% normal goat serum) to the last visible endpoint or a 1:2,048 dilution. For the detection of IgM, IgG was first absorbed with a Mini Rapi-Sep-M kit (Panbio Diagnostics, Columbia, MD) according to the manufacturer's instructions. Fluorescein isothiocyanate–labeled goat anti-dog IgM (anti-μ chain) and anti-dog IgG (anti-γ chain) (Kirkegaard and Perry Laboratories, Gaithersburg, MD) were diluted 1:100 and 1:150, respectively. IgG and IgM titers ≥ 1:64 were considered positive.
Protein gel electrophoresis and Western blotting.
Solubilized lysates of rickettsiae (15 μg at a concentration of 1.5 mg/mL) were loaded onto 12.5% sodium dodecyl sulfate–polyacrylamide gels, pH 8.8, with a 3% stacking gel, pH 6.8. Proteins were separated by electrophoresis at 3 mA for 1 hour through the stacking gel and at 12 mA for 2 hours through the separating gel at room temperature in a Mini-Protein Gel chamber (Bio-Rad, Hercules, CA) as described.30
Absorption antigens were prepared by solubilization of the Rickettsia-infected fraction of Vero cells (same stocks as used with IFA testing) using CelLyticTM B II Bacterial Cell Lysis Reagent (Sigma, St. Louis, MO) supplemented with lysozyme and protease inhibitor cocktail according to the manufacturer's instructions. Protein concentration was measured by using the bicinchoninic acid protein assay (Pierce, Rockford, IL) and normalized to a concentration of 1 mg/mL of the estimated rickettsial protein content. Serum absorptions were performed overnight at room temperature31 by using the solubilized nuclear fractions. Unabsorbed and absorbed serum specimens were incubated for 1.5 hours with membranes at final dilutions of 1:200–1:10,000 in 2% nonfat dry milk in TTBS buffer. To detect specific antigen–antibody complex formation, peroxidase-conjugated goat anti-dog IgG (heavy plus light chain; Kirkegaard and Perry Laboratories) diluted 1:1,000 in 2% nonfat dry milk in TTBS buffer were incubated with the membrane for 1.5 hours. Bound enzyme was detected by reaction with substrate solution containing 0.015% 4-chloro-1-naphthol (Bio-Rad) and 0.015% hydrogen peroxide.
Tick collection and processing.
Ticks were collected from the dogs by the primary care veterinarian and by the dog owner from the property and sent to the CDC by LACVPH for further testing (Table 1). Ticks were identified to species by using standard taxonomic keys. Molecular identification of selected ticks was performed by sequencing of a fragment of the 12S mitochondrial ribosomal RNA gene as described.32
Table 1.
Detection of persistent presence of spotted fever group rickettsiae in Rhipicephalus sanguineus, California*
| Date collected | Source of ticks | No. collected | Positive by SYBR Green PCR | No. sequenced | Rickettsial prevalence, % | ||
|---|---|---|---|---|---|---|---|
| Total PCR positive | Female (no. positive/no. tested) | Male (no. positive/no. tested) | |||||
| 3/24/2008 | Dog B | 15 | 12 | 9/12 | 3/3 | 5 | 80 |
| 4/3/2008 | Property yard | 50 | 32 | 2/2 | 30/48 | 4 | 64 |
| 4/13/2009 | Dog A | 23 | 0 | 0/11 | 0/12 | 0 | 0 |
| 4/16/2009 | Dog A | 48 | 17 | 9/24 | 8/24 | 8 | 35 |
| 4/17/2009 | Dog C | 31 | 6 | 1/8 | 5/23 | 1 | 19 |
| 4/20/2009 | Dog C | 155 | 35 | 4/40 | 31/115 | 12 | 23 |
| 4/20/2009 | Dog D | 10 | 3 | 1/5 | 2/5 | 1 | 30 |
PCR = polymerase chain reaction.
PCR testing and sequence analysis.
DNA was extracted from organ tissues of dog A and whole blood samples by using the QIAamp DNA Mini Kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions and stored at 4°C before analysis. Canine DNA samples were tested by nested PCR that amplified the 17-kD protein gene and by SYBR Green PCR specific for an outer membrane protein A (ompA) gene fragment.30,33
Four microliters of DNA extracted from individual ticks was tested for SFGR by using the ompA SYBR Green PCR assay as described.33 Genomic DNA of R. slovaca and R. sibirica were run on every plate as positive controls; four no template controls were included on each plate to ensure that there was no cross-contamination of reagents. All samples were tested as two replicates, and melting curves were analyzed for each amplicon by using an iCycler (Bio-Rad). For all PCR-positive ticks a 70–602 nucleotide fragment of ompA was amplified by semi-nested PCR,33 purified, and sequenced by using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) according to the manufacturer's recommendations on an ABI 3130xl genetic analyzer. Sequencing reads were assembled by using Sequencher 4.8 (Gene Codes, Ann Arbor, MI). Homologous sequences were detected by using the National Center for Biotechnology Information (Bethesda, MD) Basic Local Alignment Sequence Tool search engine.
Results
Description of canine cases and the study site.
Dog A, a German shepherd, was brought to a local veterinarian on July 22, 2007, August 26, 2007, and April 13, 2009, with episodes of anorexia, lethargy, leukopenia, increased levels of liver enzymes, and heavy Rh. sanguineus infestation. Seropositivity to SFGR was established by using R. rickettsii antigen and a commercial IFA (titer = 1:128) after the second episode of illness. Oral doxycycline was prescribed and resulted in complete recovery. Doxycycline administered after the third episode resulted in minimal improvement and the dog was humanely killed because of its continued poor health. Dog B, a Doberman pinscher mixed breed, had similar tick infestation and mild illness, accompanied by fever and IgG titers to R. rickettsii antigen (titer = 1:512) in March 2008.
The dog owner used a pyrethrin-shampoo bath and topical fipronil for animal treatment and amitraz-impregnated collars for all dogs, and pyrethroid-based spray for environmental tick control in August 2007, and reported successful results until efforts ceased in 2008. Tick numbers then began to increase considerably again on the dogs by late spring of 2009.
Tick testing results.
All ticks collected from dogs and from the property were morphologically identified as Rh. sanguineus; their 12S mitochondrial rDNA (HM014443) had the highest nucleotide sequence identity to the homologous gene of Rh. sanguineus from Oklahoma (AF081829) and the Mediterranean region (AF150020 and AF150018).
Thirty-two of 50 questing Rh. sanguineus collected from the property yard in April 2008 were SYBR Green PCR positive for SFGR DNA (Table 1). The ompA fragment (70–602 nucleotides) was amplified by semi-nested PCR and sequenced from four PCR-positive tick DNAs. Only one ompA genetic type was identified (HM014444); amplicons sequenced were identical to each other and had 99% and 100% sequence similarity, respectively, to the homologous fragments of R. massiliae AZT80 and Bar29. The rest of the 50 questing tick samples tested had relatively low copy numbers of the rickettsial DNA (cycle threshold > 36.9), which precluded amplification of the larger ompA fragment required for sequence identification of the SFGR. Twelve of 15 Rh. sanguineus collected from dog B in late March 2008 were PCR positive for SFGR DNA. Nucleotide sequence of the five amplicons analyzed had 100% sequence identity to the homologous ompA fragment of R. massiliae Bar29.
After interruption in acaricide applications, tick infestation of all dogs was resurgent in 2009. Five separate collections of 10–155 ticks were made from the dogs (mean = 53.4 ticks/collection, median = 31 ticks/collection, n = 267). SFGR DNA was detected in 4 of 5 tick collections (61 of 267) (Table 1). The PCR-positive ticks were found on each of the four dogs with a prevalence ranging from 19% to 35% (mean = 26.75%). Nucleotide sequences of the 70–602 nucleotide fragment of ompA amplicons analyzed were all identical to those of R. massiliae Bar29.
Serologic and PCR evaluation of dogs.
Serum specimens collected from all four dogs demonstrated broad IFA reactivity for IgM and IgG against antigens from each of the 4 SFGR (Table 2). Antibody titers ≥ 1:64 to each of the SFGR antigens were identified in one or more serum samples from each dog evaluated. Dog A had a previous history of illness and coincident increased R. rickettsii IgG titers tested by a commercial laboratory in 2007. In 2009, dogs A, C, and D had IgG titers to R. massiliae much higher than cross-reactive IgG titers to R. rhipicephali, R. rickettsii, and 364D Rickettsia.
Table 2.
Detection of antibodies to spotted fever group Rickettsia antigens in canine sera, California*
| Dog | Date of serum collection | Reciprocal IgG (IgM) IFA titer to antigen | |||
|---|---|---|---|---|---|
| R. massiliae AZT80 | R. rhipicephali 3-7-♀-6 | Rickettsia 364D | R. rickettsii Bitterroot | ||
| A | 8/26/2007 | 256 (128) | 256 (128) | 128 (256) | 1,024 (512) |
| 8/28/2007 | 128 (128) | 128 (256) | 128 (512) | 256 (256) | |
| 4/13/2009 | 1,024 (< 64) | 256 (128) | 128 (256) | 128 (128) | |
| 4/21/2009 | 128 (< 64) | < 64 (< 64) | < 64 (128) | < 64 (< 64) | |
| B | 3/24/2008 | 1,024 (512) | 1,024 (2,048) | 1,024 (> 2048) | > 2,048 (256) |
| 5/15/2008 | 512 (128) | 128 (2,048) | 512 (512) | 1,054 (256) | |
| 4/20/2009 | 1,024 (128) | > 2,048 (64) | > 2,048 (512) | > 2,048 (64) | |
| C | 4/17/2009 | > 2,048 (128) | 1,024 (256) | 128 (128) | 512 (128) |
| D | 4/20/2009 | > 2,048 (256) | 1,024 (512) | 256 (256) | 256 (128) |
IFA = indirect immunofluorescent antibody.
None of the blood samples obtained from the dogs on the day of their presentation to the veterinarian and necropsy samples of dog A, including brain, lung, heart, liver, spleen, mesenteric lymph node and adrenal gland, were PCR positive for SFGR. No useful etiologic observations were made after hematoxylin eosin staining and immunohistochemical staining for SFGR in postmortem tissues of dog A.
Identification of rickettsial agents responsible for seroconversion by Western blotting and serum cross-absorption.
Serum samples from all four dogs were tested by Western blotting with four SFGR antigens and showed strong reactivity with high molecular mass proteins ranging from 116 kD to 127 kD (Figure 1A). Samples also contained antibodies reacting with low molecular mass proteins and lipopolysachharide antigens. However, their detection and visualization were more variable and dependent on the serum working dilution tested. To evaluate the nature of the rickettsial agent responsible for seroconversion, serum absorption with each of four SFGR was performed and each of the depleted sera was tested side-by-side with diluent only–treated homologous serum samples at the same working dilution. Similar absorption results were obtained with serum from each of the four dogs, but are shown only for dog B serum. When absorption was performed with R. massiliae antigen (Figure 1B), it resulted in removal and absence of binding of homologous and heterologous SFGR antibodies from serum samples treated and negative results by Western blotting (Figure 1A). In contrast, when absorption was performed with R. rhipicephali (Figure 1C), 364D Rickettsia (Figure 1D), or R. rickettsii (Figure 1E) antigens, only cross-reactive antibodies to homologous antigens were removed, and reactivity of antibodies to R. massiliae was still detectable.
Figure 1.
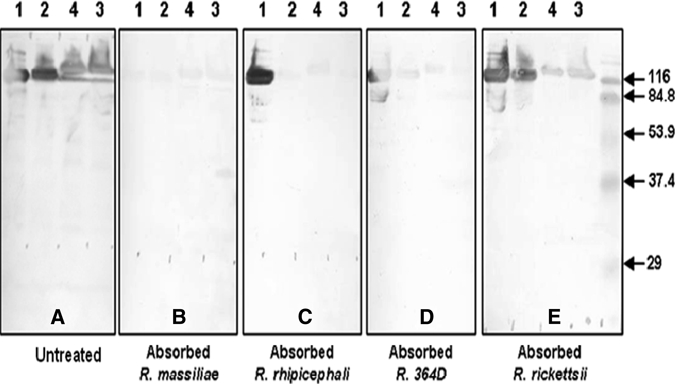
Western blotting assay and cross-absorption studies on serum from dog B, California. Western blotting was performed with serum collected on 4/20/2009 and 15 μg of rickettsial protein per lane (A). Treatment of serum with antigen diluents (A), and Rickettsia massiliae (B), R. rhipicephali (C), 364D Rickettsia (D) and R. rickettsii (E) antigens, followed by Western blotting on the resulting treated serum samples, was performed according to the procedure described in the Materials and Methods. Antigens were loaded on the gel in the following order: lane1, R. massiliae AZT-80; lane 2, R. rhipicephali CA871; lane 3, 364D Rickettsia; lane 4, R. rickettsii Bitterroot. Positions of prestained broad range sodium dodecyl sulfate-polyacrylamide gel electrophoresis standards (Bio-Rad, Hercules, CA) are indicated on the sides of the blots. All serum samples were tested by Western blotting at a final dilution of 1:5,000.
Discussion
To our knowledge, this is the first report of R. massiliae in Rh. sanguineus from California. Our findings expand the known geographic range in North America for R. massiliae, which now includes southern California in addition to a reported focus in Arizona.5 Numerous ticks containing R. massiliae were present on this property during 2008–2009.
Canine infection with R. conorii (the etiologic agent of Mediterranean spotted fever) shows clinical symptoms and serum and hematologic abnormalities similar to those of Rocky Mountain spotted fever.34 In both infections, rickettsiemia detectable between days 2 and 12 after inoculation develops in acutely ill dogs. This illness is followed by complete clearance and development of anti-rickettsial IgG; its persistence and titers depend on the number of inoculated rickettsiae.35,36 In contrast, dogs infected with R. montanensis, a SFGR of unknown pathogenicity, remain asymptomatic. However, such exposure is usually sufficient to elicit protective immune response to subsequent inoculation with R. rickettsii.37 Antibody responses in dogs infected with R. rickettsii show a similar pattern of reactivity to R. rickettsii, R. montanensis, R. rhipicephali, and R. bellii. However, treatment with tetracycline causes significant delays in serologic responses of infected dogs to heterologous rickettsial species.37
Two of the four dogs had an illness compatible with mild-to-moderate canine rickettsioses caused by R. rickettsii and R. conorii.34,38,39 Although the two other dogs were never reported to be ill, their serologic evaluation confirmed strong seropositivity to SFGR. The repeated consistent detection of R. massiliae in engorged and questing Rh. sanguineus and serum cross-absorption Western blotting results implicate R. massiliae as the likely etiologic agent responsible for SFGR seropositivity detected in all four dogs. Because only whole blood samples from immune dogs were collected and tested, it is not surprising that PCR detection of rickettsial DNA was not observed. Therefore, subsequent episodes of febrile illness in dog A were probably not caused by repeated and persistent exposure to R. massiliae-infected ticks. Although canine susceptibility to R. massiliae has not been established experimentally or proven clinically, this human pathogen may also cause rickettsial infection in dogs. Furthermore, immune response to R. massiliae will probably prevent infection of dogs with other SFGR.
Natural maintenance of SFGR depends in part on the efficiency of transovarial and transstadial transmission in their tick vectors. However, this vector–agent interaction appears to vary significantly among different rickettsial species and ticks. Our observations demonstrated inefficient and damaging vertical transmission of R. rickettsii in Rh. sanguineus from eastern Arizona.40 In contrast, R. massiliae Mtu1 shows efficient transovarial and transstadial transmission in Rh. turanicus, excretion of R. massiliae in saliva and feces of infected ticks, and transmission through co-feeding to Rh. sanguineus.41 High rates of R. massiliae infections were demonstrated in Rh. sanguineus collected from dogs in the current study (19–80% prevalence in different collections), in questing Arizona ticks from one household used for isolation of R. massiliae AZT80 (25% of 20),5,40 and in ticks from dogs in Buenos Aires (21.5% of 107).18 Because infection with this agent was not detected in any of 6242 and 164 (Eremeeva ME, unpublished data) Rh. sanguineus from two other sites in California, it appears that high prevalence rates of R. massiliae infections in ticks may arise either from clonal expansion from a single infected female tick or by efficient co-feeding transmission of this agent between ticks in a restricted focus.
Dogs evaluated during this study had high titers of IgG antibody reactive with R. massiliae. Similarly, 80% of 152 dogs infested with R. massiliae-infected Rh. sanguineus in Buenos Aires had antibodies reactive with R. conorii.18 However, a high percentage of ticks removed from seroconverted dogs were PCR positive in both studies, which suggested that antibodies against SFGR in the dogs did not affect significantly either transovarial and transstadial transmission or co-feeding acquisition of R. massiliae. How general this finding may be or which route is impacted more severely by the presence of antibodies in dogs is being evaluated in controlled laboratory studies.
Rhipicephalus sanguineus is found throughout the United States. Although it is commonly stated that this tick does not bite humans in this country, there are multiple reports of human exposures to Rh. sanguineus.43–45 Such human encounters may become more frequent when severe infestations occur.46 High temperatures are thought to increase the risk of humans being bitten.20 In temperate climates, Rh. sanguineus may establish persistent infestations in the environment and also can penetrate inside houses.47,48
Common steps for prevention of most Ixodid tick exposures, which include avoiding tick-infested areas and wearing protective clothing, are not sufficient for addressing nidicolous Rh. sanguineus infestations. Such infestations may also be expensive to eliminate and may recur (as in this report) after partial attempts at eradication. Routine canine inspections and use of shampoos and tick treatments are effective approaches, but persisting infestations require environmental control measures, including the services of professional exterminators. Households with fewer resources may be less able to control such infestations. Homeless persons surrounded by dogs are especially vulnerable to bites from Rh. sanguineus.49
Physicians and veterinarians are encouraged to maintain skills on basic tick identification, to differentiate Rh. sanguineus ticks from other Ixodid ticks, and to counsel patients and dog owners on tick control. Physicians in California and elsewhere should consider R. massiliae as a potential cause of illness in their patients, especially in those exposed to dogs and Rh. sanguineus.
ACKNOWLEDGMENTS
We thank Gail Van Gordon and Robyn Spano for facilitating this collaboration and for help with specimen storage, Karen Ehnert and Alexandra Swanson for reviewing the manuscript, Sarah Janis and Christopher Tsai for assistance with collection of specimens, Shari Lydy for membrane protein solubilization protocol, Christopher D. Paddock for performing immunohistochemical testing, and Peggy Barr and Linda Kidd for helping set up local surveillance for spotted fever group rickettsiae in dogs. We also thank the owner of the dogs for interest in this study and permission to perform testing.
Disclaimer: The findings and conclusions are those of the authors and do not necessarily reflect the views of the U.S. Department of Health and Human Services.
Footnotes
Authors' addresses: Emily Beeler, Veterinary Public Health and Rabies Control Program, Los Angeles County Department of Public Health, Los Angeles, CA, E-mail: ebeeler@ph.lacounty.gov. Kyle F. Abramowicz, Maria L. Zambrano, Michele M. Sturgeon, Gregory A. Dasch, and Marina E. Eremeeva, Rickettsial Zoonoses Branch, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: kabramowicz@cdc.gov, mzambrano@cdc.gov, msturgeon@cdc.gov, gdasch@cdc.gov, and meremeeva@cdc.gov. Nada Khalaf, VCA McClave Animal Hospital, Reseda, CA, E-mail: nada.khalaf@vcahospitals.com. Renjie Hu, Vector-Borne Disease Section, Center for Infectious Diseases, California Department of Public Health, Ontario, CA, E-mail: renjie.hu@cdph.ca.gov.
References
- 1.Beati L, Finidori JP, Gilot B, Raoult D. Comparison of serologic typing, sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein analysis, and genetic restriction fragment length polymorphism analysis for identification of rickettsiae: characterization of two new rickettsial strains. J Clin Microbiol. 1992;30:1922–1930. doi: 10.1128/jcm.30.8.1922-1930.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beati L, Raoult D. Rickettsia massiliae sp. nov., a new spotted fever group Rickettsia. Int J Syst Bacteriol. 1993;43:839–840. doi: 10.1099/00207713-43-4-839. [DOI] [PubMed] [Google Scholar]
- 3.Babalis T, Tselentis Y, Roux V, Psaroulaki A, Raoult D. Isolation and identification of a rickettsial strain related to Rickettsia massiliae in Greek ticks. Am J Trop Med Hyg. 1994;50:365–372. doi: 10.4269/ajtmh.1994.50.365. [DOI] [PubMed] [Google Scholar]
- 4.Beati L, Roux V, Ortuno A, Castella J, Porta FS, Raoult D. Phenotypic and genotypic characterization of spotted fever group Rickettsiae isolated from Catalan Rhipicephalus sanguineus ticks. J Clin Microbiol. 1996;34:2688–2694. doi: 10.1128/jcm.34.11.2688-2694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eremeeva M, Bosserman E, Demma L, Zambrano M, Blau D, Dasch G. Isolation and identification of Rickettsia massiliae from Rhipicephalus sanguineus ticks collected in Arizona. Appl Environ Microbiol. 2006;72:5569–5577. doi: 10.1128/AEM.00122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupont HT, Cornet JP, Raoult D. Identification of rickettsiae from ticks collected in the Central African Republic using the polymerase chain reaction. Am J Trop Med Hyg. 1994;50:373–380. doi: 10.4269/ajtmh.1994.50.373. [DOI] [PubMed] [Google Scholar]
- 7.Bernasconi M, Casati S, Peter O, Piffaretti J-C. Rhipicephalus ticks infected with Rickettsia and Coxiella in southern Switzerland (Canton Ticino) Inf Gen Evol. 2002;2:111–120. doi: 10.1016/s1567-1348(02)00092-8. [DOI] [PubMed] [Google Scholar]
- 8.Bacellar F, Regnery RL, Nuncio MS, Filipe AR. Genotypic evaluation of rickettsial isolates recovered from various species of ticks in Portugal. Epidemiol Infect. 1995;114:169–178. doi: 10.1017/s095026880005202x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bitam I, Parola P, Matsumoto K, Rolain JM, Baziz B, Boubidi SC, Harrat Z, Belkaid M, Raoult D. First molecular detection of R. conorii, R. aeschlimannii, and R. massiliae in ticks from Algeria. Ann N Y Acad Sci. 2006;1078:368–372. doi: 10.1196/annals.1374.073. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Soto P, Perez-Sanchez R, Diaz Martin V, Encinas-Grandes A, Alamo Sanz R. Rickettsia massiliae in ticks removed from humans in Castilla y León, Spain. Eur J Clin Microbiol Infect Dis. 2006;25:811–813. doi: 10.1007/s10096-006-0217-9. [DOI] [PubMed] [Google Scholar]
- 11.Marquez F. Spotted fever group Rickettsia in ticks from southeastern Spain natural parks. Exp Appl Acarol. 2008;45:185–194. doi: 10.1007/s10493-008-9181-7. [DOI] [PubMed] [Google Scholar]
- 12.Marquez FJ, Rodriguez-Libana JJ, Soriguer RC, Munian MA, Bernabeu-Wittel M, Caruz A, Contreras-Chova F. Spotted fever group Rickettsia in brown dog ticks Rhipicephalus sanguineus in southwestern Spain. Parasitol Res. 2008;103:119–122. doi: 10.1007/s00436-008-0938-z. [DOI] [PubMed] [Google Scholar]
- 13.Psaroulaki A, Ragiadakou D, Kouris G, Papadopoulos B, Chaniotis B, Tselentis Y. Ticks, tick-borne rickettsiae, and Coxiella burnetii in the Greek Island of Cephalonia. Ann N Y Acad Sci. 2006;1078:389–399. doi: 10.1196/annals.1374.077. [DOI] [PubMed] [Google Scholar]
- 14.Santos-Silva M, Sousa R, Santos A, Melo P, Encarnao V, Bacellar F. Ticks parasitizing wild birds in Portugal: detection of Rickettsia aeschlimannii, R. helvetica and R. massiliae. Exp Appl Acarol. 2006;39:331–338. doi: 10.1007/s10493-006-9008-3. [DOI] [PubMed] [Google Scholar]
- 15.Sarih M, Socolovschi C, Boudebouch N, Hassar M, Raoult D, Parola P. Spotted fever group rickettsiae in ticks, Morocco. Emerg Infect Dis. 2008;14:1067–1073. doi: 10.3201/eid1407.070096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berrelha J, Briolant S, Muller F, Rolain JM, Marie JL, Pagés F, Raoult D, Parola P. Rickettsia felis and Rickettsia massiliae in Ivory Coast, Africa. Clin Microbiol Infect. 2009;15:251–252. doi: 10.1111/j.1469-0691.2008.02273.x. [DOI] [PubMed] [Google Scholar]
- 17.Harrus S, Perlman-Avrahami A, Mumcuoglu KY, Morick D, Baneth G. Molecular detection of Rickettsia massiliae, Rickettsia sibirica mongolitimonae and Rickettsia conorii israelensis in ticks from Israel. Clin Microbiol Infect. 2010 doi: 10.1111/j.1469-0691.2010.03224.x. Mar 20 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Cicuttin GL, Rodríguez-Vargas M, Jado I, Anda P. Primera detección de Rickettsia massiliae en la ciudad de Buenos Aires. Resultados preliminaries. Rev Argentina Zoon. 2004;1:8–10. [Google Scholar]
- 19.Vitale G, Mansuelo S, Rolain J-M, Raoult D. Rickettsia massiliae human isolation. Emerg Infect Dis. 2006;12:174–175. doi: 10.3201/eid1201.050850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parola P, Socolovschi C, Jeanjean L, Bitam I, Fournier P-E, Sotto A, Labauge P, Raoult D. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Negl Trop Dis. 2008;2:e338–e338. doi: 10.1371/journal.pntd.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Garcia J, Portillo A, Nez M, Santibez S, Castro B, Oteo J. A patient from Argentina infected with Rickettsia massiliae. Am J Trop Med Hyg. 2010;82:691–692. doi: 10.4269/ajtmh.2010.09-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bella F, Espejo E, Uriz S, Serrano JA, Alegre MD, Tort J. Randomized trial of 5-day rifampin versus 1-day doxycycline therapy for Mediterranean spotted fever. J Infect Dis. 1991;164:433–434. doi: 10.1093/infdis/164.2.433. [DOI] [PubMed] [Google Scholar]
- 23.Rolain JM, Maurin M, Vestris G, Raoult D. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother. 1998;42:1537–1541. doi: 10.1128/aac.42.7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pons I, Nogueras MM, Sanfeliu I, Ortuno A, Segura F. Serological evidence of infection with Bar29 in dog population of Catalonia, in the northeast of Spain. 5th International Meeting on Rickettsiae and Rickettsial Diseases. Marseille; France: 2008. Abstract Book. [Google Scholar]
- 25.Ortuno A, Pons I, Nogueras MM, Castella J, Segura F. The dog as an epidemiological marker of Rickettsia conorii infection. Clin Microbiol Infect. 2009;15:241–242. doi: 10.1111/j.1469-0691.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 26.Paddock C, Brenner O, Vaid C, Boyd DB, Berg J, Joseph R, Zaki S, Childs J. Short report: concurrent Rocky Mountain spotted fever in a dog and its owner. Am J Trop Med Hyg. 2002;66:197–199. doi: 10.4269/ajtmh.2002.66.197. [DOI] [PubMed] [Google Scholar]
- 27.Elchos B, Goddard J. Implications of presumptive Rocky Mountain spotted fever in two dogs and their owner. J Am Vet Med Assoc. 2003;223:1450–1452. doi: 10.2460/javma.2003.223.1450. 1433. [DOI] [PubMed] [Google Scholar]
- 28.Paddock C, Koss T, Eremeeva M, Dasch G, Zaki S, Sumner J. Isolation of Rickettsia akari from eschars of patients with rickettsialpox. Am J Trop Med Hyg. 2006;75:732–738. [PubMed] [Google Scholar]
- 29.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Eremeeva ME, Balayeva NM, Ignatovich VF, Raoult D. Proteinic and genomic identification of spotted fever group rickettsiae isolated in the former USSR. J Clin Microbiol. 1993;31:2625–2633. doi: 10.1128/jcm.31.10.2625-2633.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensenius M, Fournier P-E, Vene S, Ringertz S, Myrvang B, Raoult D. Comparison of immunofluorescence, Western blotting, and cross-adsorption assays for diagnosis of African tick bite fever. Clin Diagn Lab Immunol. 2004;11:786–788. doi: 10.1128/CDLI.11.4.786-788.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beati L, Keirans JE. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol. 2001;87:32–48. doi: 10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Wikswo M, Hu R, Dasch G, Krueger L, Arugay A, Jones K, Hess B, Bennett S, Kramer V, Eremeeva M. Detection and identification of spotted fever group rickettsiae in Dermacentor species from southern California. J Med Entomol. 2008;45:509–516. doi: 10.1603/0022-2585(2008)45[509:daiosf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Solano-Gallego L, Kidd L, Trotta M, Di Marco M, Caldin M, Furlanello T, Breitschwerdt E. Febrile illness associated with Rickettsia conorii infection in dogs from Sicily. Emerg Infect Dis. 2006;12:1985–1988. doi: 10.3201/eid1212.060326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly PJ, Mattheaman LA, Mason PR, Courtney S, Katsande C, Rukwava J. Experimental infection of dogs with a Zimbabwean strain of Rickettsia conorii. J Trop Med Hyg. 1992;95:322–326. [PubMed] [Google Scholar]
- 36.Norment BR, Burgdorfer W. Susceptibility and reservoir potential of the dog to spotted fever group rickettsiae. Am J Vet Res. 1984;45:1706–1710. [PubMed] [Google Scholar]
- 37.Breitschwerdt EB, Walker DH, Levy MG, Burgdorfer W, Corbett WT, Hurlbert SA, Stebbins ME, Curtis BC, Allen DA. Clinical, hematologic, and humoral immune response in female dogs inoculated with Rickettsia rickettsii and Rickettsia montana. Am J Vet Res. 1988;49:70–76. [PubMed] [Google Scholar]
- 38.Greene CE, Breitschwerdt EB. In: Infectious Diseases of the Dog and Cat. Green CE, editor. St. Louis: Saunders-Elsevier; 2006. pp. 232–245. (Rocky Mountain spotted fever, murine typhuslike disease, rickettsialpox, typhus, and Q fever). [Google Scholar]
- 39.Labruna M, Kamakura O, Moraes-Filho J, Horta M, Pacheco R. Rocky Mountain spotted fever in dogs, Brazil. Emerg Infect Dis. 2009;15:458–460. doi: 10.3201/eid1503.081227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eremeeva ME, Moriarity J, Bosserman EA, Zambrano ML, Nicholson WL, Paddock CD, Demma L, Traeger M, Johnson B, Dickson J, Levin ML, Swerdlow D, McQuiston JH, Dasch GA. Detection of rickettsiae in Rhipicephalus sanguineus ticks from Arizona. 20th Meeting of the American Society for Rickettsiology and the 5th International Conference on Bartonella as Emerging Pathogens. Asilomar; CA: 2006. [Google Scholar]
- 41.Matsumoto K, Ogawa M, Brouqui P, Raoult D, Parola P. Transmission of Rickettsia massiliae in the tick, Rhipicephalus turanicus. Med Vet Ent. 2005;19:263–270. doi: 10.1111/j.1365-2915.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- 42.Wikswo M, Hu R, Metzger M, Eremeeva M. Detection of Rickettsia rickettsii and Bartonella henselae in Rhipicephalus sanguineus ticks from California. J Med Entomol. 2007;44:158–162. doi: 10.1603/0022-2585(2007)44[158:dorrab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Carpenter TL, McMeans MC, McHugh CP. Additional instances of human parasitism by the brown dog tick (Acari: Ixodidae) J Med Entomol. 1990;27:1065–1066. doi: 10.1093/jmedent/27.6.1065. [DOI] [PubMed] [Google Scholar]
- 44.Felz MW, Durden LA, Oliver JH. Ticks parasitizing humans in Georgia and South Carolina. J Parasitol. 1996;82:505–508. [PubMed] [Google Scholar]
- 45.Goddard J. Focus of human parasitism by the brown dog tick, Rhipicephalus sanguineus (Acari: Ixodidae) J Med Entomol. 1989;26:628–629. doi: 10.1093/jmedent/26.6.628. [DOI] [PubMed] [Google Scholar]
- 46.Demma L, Traeger M, Nicholson W, Paddock C, Blau D, Eremeeva M, Dasch G, Levin M, Singleton J, Zaki S, Cheek J, Swerdlow D, McQuiston J. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- 47.Fox MT, Sykes TJ. Establishment of the tropical dog tick, Rhipicephalus sanguineus, in a house in London. Vet Rec. 1985;116:661–662. doi: 10.1136/vr.116.25.661. [DOI] [PubMed] [Google Scholar]
- 48.Uspensky I, Ioffe-Uspensky I. The dog factor in brown dog tick Rhipicephalus sanguineus (Acari: Ixodidae) infestations in and near human dwellings. Int J Med Microbiol. 2002;291((Suppl 33)):156–163. doi: 10.1016/s1438-4221(02)80030-3. [DOI] [PubMed] [Google Scholar]
- 49.Brouqui P, Raoult D. Arthropod-borne diseases in homeless. Ann N Y Acad Sci. 2006;1078:223–235. doi: 10.1196/annals.1374.041. [DOI] [PubMed] [Google Scholar]


