Abstract
Miltefosine has been used in the treatment of several new world cutaneous leishmaniasis (CL) species with variable efficacy. Our study is the first evidence on its clinical efficacy in Leishmania (Viannia) guyanensis. In this phase II/III randomized clinical trial, 90 CL patients were randomly allocated (2:1) to oral miltefosine (2.5 mg/kg/day/28 days) (N = 60) or parenteral antimony (15–20 mg/Sb/kg/day/20 days) (N = 30) according to age groups: 2–12 y/o and 13–65 y/o. Patients were human immunodeficiency virus (HIV) noninfected parasitological proven CL without previous treatment. Definitive cure was accessed at 6 months follow-up visit. No severe adverse events occurred. Vomiting was the most frequent adverse event (48.3%) followed by nausea (8.6%) and diarrhea (6.7%). Cure rates were 71.4% (95% confidence interval [CI] = 57.8–82.7) and 53.6% (95% CI = 33.9–72.5) (P = 0.05) for miltefosine and antimonial, respectively. There were no differences in cure rates between age groups within the same treatment arms. Miltefosine was safe and relatively well tolerated and cure rate was higher than antimony.
Introduction
Antimonials are still the first-line drugs for the treatment of cutaneous leishmaniasis (CL) in Brazil where Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis are the main species causing human disease.1–3 Miltefosine (Impavido), a phosphatidylcholine analogue, is the first clinically active oral antileishmanial drug commercially available for the treatment of both visceral and CL.4,5 The drug was recently (2005–2006) registered for treatment of leishmaniasis in Colombia, Guatemala, Honduras, and Ecuador. The evidence of its clinical efficacy for new world CL relies on four studies performed by Soto and others.6–9
The first study was an open-label phase I/II dose escalation trial of miltefosine in 72 male Colombian soldiers (mean weight 67 kg) with CL. Doses varied from 50 mg/day/for 20 days to 150 mg/day for 28 days. There was a dose-response trend with cure rates varying from 64% to 89%. Unfortunately, leishmania cultures were available in only 15/72 (21%) patients: 5 isolates were identified as Leishmania (Leishmania) amazonensis and 10 as Leishmania (Viannia) panamensis.6
The second study was a confirmatory placebo-controlled trial for CL conducted in two sites: Colombia and Guatemala. In Colombia, the cure rate was 81.6% (37.5% for placebo) and in Guatemala 55% (25% for placebo). Patients (> 12 y/o) in either sites received miltefosine 100 mg/day (weight ≤ 45 kg) or 150 mg/day (weight ≥ 45 kg) for 28 days. Species identification was available in 7/74 (9.5%) Colombian patients all identified as L. (V.) panamensis and in 46/60 (76.7%) patients in Guatemala: 29 (63%) identified as L. (V.) braziliensis and 17 (37%) as Leishmania (Leishmania) mexicana.7
The third study was a non-randomized controlled trial in 97 Bolivian patients with mucosal leishmaniasis: 78 adult patients (mean weight 60 kg ± 10 kg) received miltefosine (2.5–3.3 mg/kg/day for 28 days), and 19 patients received 45 doses of amphotericin B (1 mg/kg every other day with a maximum daily dose of 45 mg). Cure rate with amphotericin B was 50% versus 83% and 58% with miltefosine in patients with mild and more extensive disease, respectively. Leishmania speciation was available in only 7/97 (7.2%) patients all identified as L. (V.) braziliensis.8
The fourth study was also performed in Bolivia in patients with ages ≥ 12 y/o with CL, was an open label, randomized 2:1 miltefosine (2.5 mg/kg/d for 28 days, N = 44) versus Glucantime (20 mg Sb+5/kg/d for 20 days, N = 18). Cure rate at 6 months was 88% for miltefosine and 94% for antimonial. No species identification was performed, although the authors stated that the main species in the study area was L. (V.) braziliensis.9 On the basis of the efficacy results of these four trials, it is clear that miltefosine-induced cure rates for new world leishmaniasis vary according to both the species of leishmania causing disease and between the same species acquired from different endemic areas. This observation was indeed pointed out in two other studies conducted in Peru where leishmania species were associated with different antimonial treatment outcomes.10,11 This variation in cure rates has also been demonstrated in patients infected with L. (V.) braziliensis and L. (V.) guyanensis in Brazil where antimonial cure rates are lower than the ones reported in Bolivia and Peru.9,12,13 Despite the epidemiologic importance of L. (V.) guyanensis in the Brazilian Amazon, only one clinical trial addressing treatment response of this leishmania species to antimonials was performed in the past 10 years.14 Our study was designed to evaluate the efficacy and safety of miltefosine versus meglumine antimoniate (Glucantime) for the treatment of CL in a L. (V.) guyanensis-endemic area near the city of Manaus, AM, Brazil
Methods
Ethical review board.
Informed consent was obtained from all study participants and/or guardians before enrollment. The study protocol was reviewed and approved by the Institutional Review Board of the Fundação de Medicina Tropical - Amazonas and by the Brazilian National Council of Ethics on Research (CONEP). This study is registered with ClinicalTrials.gov identifier NCT0060054.
Randomization.
Random numbers in a 2:1 allocation for miltefosine were obtained using StataCorp LP 9 (College Station, TX).
Sample size.
The sample size was calculated assuming an expected 30% difference between groups (effectiveness of at least 50% for meglumine and 80% for miltefosine), 95% confidence interval (CI) and a power of 80%.
Study design.
This phase II/III prospective open label active-control trial was designed to evaluate the efficacy and safety of miltefosine (Impavido, Zentaris GmbH, Germany) compared with meglumine antimoniate (Glucantime, Aventis Pharma, Brazil). The study was conducted at the dermatology outpatient clinic at the Fundação de Medicina Tropical—Amazonas, Manaus, AM, Brazil.
Patients who met the entry criteria were randomly allocated (2:1) to oral miltefosine for 28 days or parenteral antimony for 20 days. In addition, patients were stratified according to age groups: 2–12 y/o and 13–65 y/o. Inclusion criteria for all patients were 1) clinical diagnosis of CL with 1–5 lesions with at least one ulcerated lesion with a diameter of 1–5 cm, 2) illness duration of less than 3 months, 3) visualization of Leishmania amastigotes on Giemsa Diff-Quick, Dade Behring, Newark, EUA stained imprint from lesion biopsies, 4) no previous leishmania treatment. Exclusion criteria were 1) evidence of immunodeficiency or antibodies to human immunodeficiency virus (HIV), 2) pregnancy or patients not willing or unable to use contraceptives during and 3 months after the end of therapy, 3) alanine aminotransferase (ALT), aspartate aminotransferase (AST) ≥ 3× normal reference values, Billirubin ≥ 2× reference values, and creatinine and blood urea nitrogen (BUN) ≥ 1.5× normal reference values, 4) any evidence of serious underlying disease (cardiac, renal, hepatic, or pulmonary) including serious infection other than CL.
Study area.
The great majority of patients were from 2 municipalities (Presidente Figueiredo and Rio Preto da Eva, located on the roads BR 174 and AM 010, ~100 km from Manaus.
Parasitology.
Parasite species identification.
Leishmania speciation was performed by amplification of the repeated heat shock protein 70 (hsp70) genes, followed by restriction fragment length polymorphism analysis (hsp70 polymerase chain reaction-restriction fragment length polymorphism [PCR-RFLP]) on skin biopsies from enrolled patients. The target genes hsp70 and mini-exon were amplified and digested as reported elsewhere.15 Restriction patterns were resolved by electrofophoresis performed in 3% gel agarose stained with ethidium bromide. Obtained patterns were compared with those of reference strains of L. (V.) braziliensis (MHOM/BZ/75/M2903), L. (V.) guyanensis (MHOM/BR/75/M4147), L. (V.) lainsoni (MHOM/PE/03/LH2443), (MHOM/PE/87/LC106), L. (L.) amazonensis (MHOM/BR/81/LTB16), and Leishmania (Viannia) naiffi (MDAS/BR/78/M5210).
Drug administration.
All study volunteers were treated as outpatients. Each patient received enough drugs for 7 days at a time. Miltefosine was supplied in blister packs with seven capsules each, containing 10 mg or 50 mg. Glucantime was supplied in vials of 5 mL containing 81 mg/Sb+5/mL.
Miltefosine was administered orally at the total target daily dosage of 2.5 mg/kg of body weight (maximum daily dose of 150 mg) for 28 consecutive days. Treatment was equally divided into two or three doses and was always given with meals according to the following weight scale:
Patients with ≤ 14 kg – total dose of 30 mg/day
Patients with ≥ 15 kg and ≤ 29 kg – total dose of 50 mg/day
Patients with ≥ 30 kg and ≤ 45 kg – total dose of 100 mg/day
Patients with ≥ 46 kg – total dose of 150 mg/day
Glucantime was administered intravenously at a dose of 20 mg Sb+5/kg/day (age group 13–65 y/o) and 15 mg Sb+5/kg/day (age group 2–12 y/o) for 20 consecutive days (maximum daily dose of 3 ampoules), according to the Ministry of Health guidelines.3
Compliance to treatments was determined as follows: Glucantime was administered at local primary health clinics and injection records kept in clinics were checked weekly by the study physician. Patients receiving miltefosine had to return the empty blister packs to receive the subsequent weekly dose. Both drugs were delivered weekly to the study site.
Study procedures.
Complete hemogram, aminotransferases (AST, ALT), blood urea, and creatinine were determined in all patients on Day 1, and weekly thereafter up to the end of therapy. Those with abnormal parameters in the last week of treatment were followed until normalization. At each weekly return for drug dispensation at the study site, patients were monitored for vomiting, diarrhea, somnolence, motion sickness, anorexia mialgia, and arthralgia. Each lesion was measured in size (two-dimensional, largest diameters in mm) at Day 1 and at each follow-up visit. A standardized digital photograph was also taken of each patient's lesions at the same time points.
Follow-up and toxicity evaluation.
Patients were seen for follow-up at 1, 2, 4, and 6 months post-therapy. Clinical and laboratory adverse events were graded according to the Common Toxicity Criteria (CTC) of the National Cancer Institute (http://ctep.cancer.gov/reporting/ctc.html).
Clinical endpoints criteria.
Partial cure: Incomplete epithelialization or incomplete regression of inflammatory
induration of one or more lesions, and no appearance of new lesions.
Apparent cure: Complete epithelialization of all ulcers and regression ≥ 70% of inflammatory indurations from all lesions.
Definite cure: Complete epithelialization of all ulcers and complete disappearance of inflammatory induration from all lesions at 6 months follow-up visit.
Clinical failure: Any of the following was classified as clinical failure:
residual lesions with presence of parasites in Giemsa Diff-Quick stained imprint, or
appearance of any new lesions, or
≥ 20% enlargement or no improvement of previously documented lesions.
If a patient fulfilled the criteria for partial cure 2 months after the end of treatment, he was classified as clinical failure and rescue treatment with antimonials or pentamidine (3 doses of 4 mg/kg IM with a maximum daily dose of 300 mg, every 48 h). The same procedure was adopted if a patient fulfilled the criteria for clinical failure at any time after the end of treatment.
Statistical analysis.
Primary study endpoints were calculated at 6 months follow-up visits (definitive cures) or when criteria defined previously were fulfilled for clinical failure. Statistical tests were performed using SAS version 9.1 (SAS Institute, Cary, NC). Comparisons between groups were done by parametric and nonparametric analysis of variance techniques as appropriate.
Results
During the 24-month study duration (Feb 2007–Dec 2008), 176 individuals with suspected CL were evaluated for study participation. Eighty-six were excluded because of the following: negative imprint for leishmania (N = 32), investigator believed they were unlikely to comply with follow-up (N = 25), refused participation (N = 15), more than 3 months of illness (N = 5), more than five lesions (N = 4), abnormal serum chemistries (N = 3) and pregnancy (N = 2). Ninety patients met the entry criteria and were enrolled in the study; 60 in the miltefosine and 30 in the antimonial treatment arm. The great majority of participants (98%) were infected patients from two CL endemic areas (Presidente Figueiredo and Rio Preto da Eva) located ~100 km from the city of Manaus. Six patients were not included in the intention to treat efficacy analysis: two patients in each treatment group were excluded because of different leishmania species and another two in the miltefosine group were excluded in the first week of treatment: one because of emigration and the other because of concomitant Plasmodium falciparum malaria. Therefore, only 84 patients were considered for drug efficacy analysis. Study compliance was very good. Only three patients were lost in follow-up: two in the miltefosine group (second- and fourth-month visits) and one in the antimonial group (fourth-month visit). Subjects enrolled were from both genders, with ages ranging from 4 to 62 years of age. There was no difference between treatment arms regarding gender, age, duration of illness, and number of lesions (Table 1).
Table 1.
Baseline characteristics of 90 patients with cutaneous leishmaniasis (CL) enrolled in the study
| Characteristics | Miltefosine (N = 60) | Glucantime (N = 30) | P value | |||
|---|---|---|---|---|---|---|
| Age group in years | ||||||
| 2–12 | 13–65 | 2–12 | 13–65 | 2–12 y/o | 13–65 y/o | |
| Male/female ratio | 13/7 | 32/8 | 7/3 | 16/4 | NA | NA |
| Age in years ± SD (range) | 9.1 ± 2.4 (4–12) | 32.5 ± 13.4 (13–62) | 8.5 ± 2.4 (4–12) | 32.3 ± 14.6 (16–59) | 0.6* | 1.0* |
| Median illness duration in days (range) | 35.7 (2–60) | 33.8 (10–60) | 33.7 (15–60) | 39.1 (10–60) | 0.7* | 0.3* |
| No. of lesions (%) | ||||||
| 1 | 13 (65) | 19 (47.5) | 7 (70) | 8 (40) | 0.8 | 0.6† |
| 2 | 5 (25) | 4 (10) | 2 (20) | 4 (20) | 1.0 | 0.4 |
| 3 | 1 (5) | 8 (20) | 1 (10) | 3 (15) | 1.0 | 0.7 |
| 4 | 1 (5) | 7 (17.5) | 0 (0) | 3 (15) | 1.0 | 1.0 |
| 5 | 0 (0) | 2 (5) | 0 (0) | 2 (10) | NA | 0·6 |
| Parasitology | ||||||
| L. (V.) guyanensis | 19 | 39 | 9 | 19 | ||
| L. (V.) braziliensis | 0 | 1 | 1 | 1 | ||
| L. (V.) lainsoni | 1 | 0 | 0 | 0 | ||
Mann-Whitney.
Binomial.
Efficacy.
Cure rates at 6 months follow-up (definite cure) were 71.4% (40/56) and 53.6% (15/28) (P = 0.05) for miltefosine and antimony, respectively (Table 2). There was no difference in cure rates in the age group 2–12 y/o among treatment arms (63.1% [12/19] versus 55.5% [5/9], P = 0.65). However, the cure in the age group 13–65 y/o was higher in the miltefosine treatment arm (75.7% [28/37] versus 52.6% [10/19], P = 0.04, 95% confidence interval [CI] = 55.8–88.2). Among the 14 patients classified as clinical failures in the miltefosine group, 3 (21.4%) never healed the leishmania lesions and 11 (78.6%) presented complete epithelialization but relapsed afterwards (apparent cure with recidiva cutis) (Figure 1). In the antimony group, 4 (33.3%) out of the 12 patients classified as clinical failures never healed and 8 (66.7%) presented complete epithelialization but also relapsed afterwards. In both treatment groups 78% of the relapses/failures occurred in the first 2 months after treatment (Table 2). Leishmania speciation was obtained in all 90 patients. L. (V.) guyanensis was identified in 86 (95.5%) patients. In three patients the identified species was L. (V.) braziliensis and in one L. (V.) lainsoni. The patient infected with L. (V.) lainsoni (miltefosine group) was cured. Two out three patients infected with L. (V.) braziliensis were treatment failures: one in each treatment group.
Table 2.
Follow-up endpoint results in both treatment groups
| Follow-up endpoints | Miltefosine | Glucantime |
|---|---|---|
| N = 56 | N = 28 | |
| End of treatment | 28 days | 20 days |
| Partial cure (%) | 37 (66.7) | 25 (89.3) |
| Apparent cure (%) | 19 (34) | 3 (10.7) |
| Failure (%) | 0 | 0 |
| Lost in follow-up (%) | 0 | 0 |
| 1 month after treatment | ||
| Partial cure (%) | 8 (14.3) | 6 (21.4) |
| Apparent cure (%) | 42 (75) | 21 (75) |
| Failure (%) | 6 (10.7) | 1 (3.6) |
| Lost in follow-up (%) | 0 | 0 |
| 2 months after treatment | ||
| Partial cure (%) | 2 (3.6) | 3 (10.7) |
| Apparent cure (%) | 41 (73.2) | 17 (60.7) |
| Failure (%) | 12 (21.4) | 8 (28.6) |
| Lost in follow-up (%) | 1 (1.8) | 0 |
| 4 months after treatment | ||
| Partial cure (%) | 0 | 0 |
| Apparent cure (%) | 40 (71.4) | 16 (57.1) |
| Failure (%) | 14 (25) | 12 (42.9) |
| Lost in follow-up (%) | 2 (3.6) | 0 |
| 6 months after treatment | ||
| Partial cure (%) | 0 | 0 |
| Definitive cure (%)* | 40 (71.4) | 15 (53.6) |
| ITT definite cure (%) | 41 (68,3) | 16 (53.3) |
| Clinical failure (%) | 14 (25) | 12 (42.9) |
| ITT clinical failure (%) | 19 (31.7) | 14 (46.7) |
| Lost in follow-up (%) | 2 (3.6) | 1 (3.5) |
| Cure rate 95% CI | 57·8–82·7 | 33·9–72·5 |
| ITT cure rate 95% CI | 55.0–79.7 | 34.3–71.7 |
ITT = intention to treat; CI = confidence interval.
P = 0.05 binomial 1-sided P = 0.08.
Figure 1.
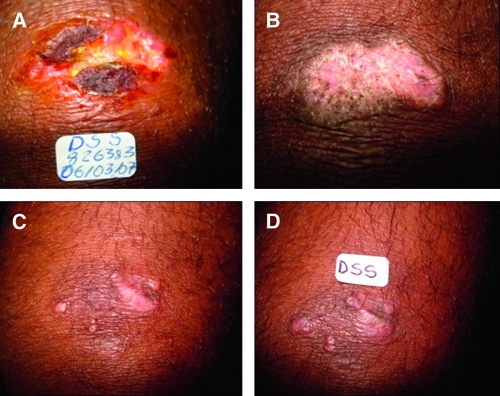
Relapse in a patient with cutaneous leishmaniasis (CL) treated with miltefosine (A), classified intially as apparent cure with complete lesion epithelialization (B) but relapse at the second month follow-up visit – recidiva cutis (C and D). Note the nodular lesions in C and D.
Toxicity and tolerability.
Study medications were generally well tolerated (Table 3). No serious adverse events (SAEs) occurred and none of the reported adverse events (AEs) required discontinuation of therapy in any patient. In the miltefosine group, clinical AEs reported were mostly related to the GI tract and occurred usually in the first week of treatment. Vomiting was the most frequent AE, reported by 48.3% (28/58) patients. Out of those 28 patients, 41% (7/17) with CTC grade 1 and 45.5% (5/11) with CTC grade 2 were children. In the second week of treatment, two patients (15 y/o and 21 y/o) developed painful testicular swelling, which resolved spontaneously within 1 week. Changes in laboratory parameters with treatment are shown in Table 4. Renal dysfunctions were uncommon and transient. Creatinine and BUN levels were above reference values in four adult patients (three with CTC grade 1 and one with CTC grade 2) at the end of treatment, which returned to normal parameters 2 weeks afterwards. Mild to moderate elevation in liver enzymes were detected in six patients and normalized after completion of therapy. In the antimony group only two AEs were detected: mild transient liver enzymes in two patients and arthralgia reported by 33% (10/30) patients.
Table 3.
Clinical toxicity data
| Symptom | Frequency % | CTC grade |
|---|---|---|
| Miltefosine (N = 58) | ||
| Vomiting* | 48.3 | 17 pts with CTC grade 1 11 pts with CTC grade 2 |
| Diarrhea† | 6.7 | 3 pts with CTC grade 1 1 pt with CTC grade 2 |
| Nausea | 8.6 | 5 pts with CTC grade 1 |
| Epigastralgia | 3.4 | 2 pts with CTC grade 1 |
| Dizziness | 3.4 | 2 pts with CTC grade 1 |
| Pruritus | 3.4 | 2 pts with CTC grade 1 |
| Orchitis‡ | 3.4 | 2 pts with CTC grade 1 |
| Glucantime (N = 30) | ||
| Arthralgias | 33 | 10 CTC grade 1 |
Vomiting: 7/17 (41%) with CTC grade 1 and 5/11 (45%) with CTC grade 2 were children (CTC grade 1: one episode in 24 hrs and a grade 2: 2–5 episodes in 24 hrs).
Diarrhea: all patients were adults (CTC grade 1: 2–3 stools/day, grade 2: 4–6 stools/day).
Two patients (15 y/o and 21 y/o) presented orchitis of 1-week duration, which resolves spontaneously.
Table 4.
Toxicity data: laboratory values at baseline and at the end of treatment*
| Parameters | Baseline mean ± SD (range) | End of treatment values |
|---|---|---|
| Miltefosine (N = 58) | ||
| Urea (15–40 mg/dL) | 23.5 ± 7.3 (6–41) | 28.5 ± 9.3 (11–55) |
| Creatinine† (0.6–1.3 mg/dL) | 0.72 ± 0.26 (0.3–1.4) | 0.95 ± 0.51 (0.2–3.6) |
| AST (15–37 U/L) | 28.2 ± 11 (16–71) | 32.8 ± 24 (13–187) |
| ALT (30–65 U/L) | 38.1 ± 11.5 (24–86) | 48.1 ± 26 (3–152) |
| Glucantime (N = 30) | ||
| Urea (15–40 mg/dL) | 23.9 ± 6.6 (8–40) | 21.9 ± 7.5 (12–38) |
| Creatinine (0.6–1.3 mg/dL) | 0.70 ± 0.22 (0.3–1.1) | 0.78 ± 0.29 (0.3–1.3) |
| AST (15–37 U/L) | 30.9 ± 17.2 (17–95) | 44.3 ± 28.7 (24–166) |
| ALT (30–65 U/L) | 41.5 ± 19.3 (25–120) | 58.6 ± 56.1 (28–318) |
AST = aspartate aminotransferase; ALT = alanine aminotransferase.
Creatinine: 4 adults (42, 44, and 56 y/o) with increase in creatinine levels (1.6, 1.7, 1.7 [CTC grade 1] and 3.6 mg/dL [CTC grade 2], respectively) in the last week of treatment. Creatinine levels returned to normal parameters at 1mo f/w.
Discussion
The data presented in this study is the first clinical evidence on efficacy of miltefosine in patients infected with L. (V.) guyanensis and also the first that included patients with CL in the age group < 12 y/o. L. (V.) guyanensis is the second most prevalent leishmania species in Brazil and probably the main species causing disease in the Brazilian Amazon above the north edge of the Amazon river. Although L. (V.) guyanensis has epidemiological importance in the Amazon region, only one treatment trial has been performed in the last 10 years, which showed that this species has a low cure rate (26.3%) with antimonials.14 Even with this low reported efficacy, the recommended treatment of CL in Brazil, regardless of the species causing the disease, remains antimonials.3 In our study, miltefosine was more effective than antimonials in the treatment of CL leishmaniasis caused by L. (V.) guyanensis (cure rate of 71.4% versus 53.6%, P = 0.05). Interestingly, 11 (78.6%) out of the 14 patients classified as clinical failures in the miltefosine group presented complete epithelialization of the lesion, but relapsed afterwards (apparent cure with recidiva cutis) (picture 2). Most relapses (86%) occurred within 2 months after the end of therapy. Although no difference in cure rates could be observed among treatment arms in the age group 2–12 y/o, a difference in efficacy was seen in the age group 13–65 y/o (75.7% [28/37] versus 52.6% [10/19], P = 0.04, 95% CI = 55.8–88.2). This difference could be explained by the smaller accumulation in plasma levels in children compared with adults.16
Miltefosine was in general well tolerated with most clinical AEs occurring in the first week of treatment and distributed equally between age groups. Vomiting was the most frequent AE, reported by 48.3% (28/58) patients followed by nausea in 8.6% (5/58) and diarrhea in 6.7% (4/58) of patients (Table 2). Of note was the presence of orchitis in two patients (15 y/o and 21 y/o) presented as a swelling painful testicle in the second week of treatment, which resolved spontaneously within 1 week of duration. This AE was never reported before in any patient treated with miltefosine. Although different in frequencies, the clinical-related AEs were also reported in the studies performed by Soto and others6–9 except that “motion sickness” was not reported by any of our patients. Laboratory AEs were mainly related to the liver and kidneys. Mild to moderate transient liver enzymes were detected in six patients. However, of more concern are the changes that occurred in kidney parameters. Four adults presented creatinine levels above 1.4 mg/dL at the end of treatment (three patients with CTC grade 1 and one patient with CTC grade 2 with creatinine of 3.6 mg/dL). However, creatinine values returned to normal parameters within 1 month of follow-up.
Antimonial cure rate was higher than the 26.3% reported by Romero and others14 for L. (V.) guyanensis in a study performed with patients from surrounding areas of the city of Manaus, located 100 km from our study's area. These differences in cure rates could be explained by a possible genetic variability among L. (V.) guyanensis resulting in different susceptibility to treatment. A study performed in the French Guiana using ribosomal fingerprinting analysis identified two distinct nonsympatric L. (V.) guyanensis populations.17 The genotype 1 was associated with higher parasite density in lesions and the need for additional treatments. Differences in L. (V.) guyanensis subpopulations were also found by Romero and others in isolates from his previous study reported previously. In this study, none of the 80 isolates reacted with the L. (V.) guyanensis monoclonal antibody B19 (considered to be specific for this species and present in isolates from French Guiana and Eastern Brazilian Amazon region).18,19 On the other hand, the parasites reacted with Mab B2 and B12, indicating that two serodemes subpopulation predominated in the Manaus region. Further studies “in vitro” and “in vivo” are needed to compare treatment response to miltefosine in antigenically distinct populations of L. (V.) guyanensis. Our study's miltefosine efficacy data, although addressing a different leishmania species, parallels with data reported in the studies performed by Soto and others.6–9 For CL acquired in Brazil, physicians should be aware that leishmania speciation may be necessary to better treat patients or tourists living in or traveling to the Brazilian Amazon. The CL caused by L. (V.) guyanensis has a low cure rate with antimonial, and miltefosine should be considered as a treatment option.
ACKNOWLEDGMENTS
We thank Erico Jamber Silva Lopes for the statistical analysis. We also thank Ralph Corey (Duke University, Durham, NC) for suggestions and the final reviewing.
Disclaimer: None of the authors of this manuscript have an association with a commercial or other entity that may pose a conflict of interest.
Footnotes
Financial support: This study is part of a National Multicenter Clinical Trial for the evaluation of miltefosine in the treatment of cutaneous leishmaniasis caused by L. (V.) guyanensis and L. (V.) braziliensis. Both studies were conducted in Brazil. The current study was funded by FINEP/Brazil (project no. 3726/05) and coordinated by the Núcleo de Medicina Tropical from the University of Brasília, Brasília, DF, Brazil. The present manuscript refers to the study arm performed at the Fundação de Medicina Tropical do Amazonas with patients infected with L. (V.) guyanensis. The other arm was conducted at the Immunology Service of the University Hospital Professor Edgar Santos at the Federal University of Bahia, on patients infected with L. (V.) braziliensis with financial support of CNPq no. 410559/2006-7 (edital mct/cnpq/ms/sctie/decit 25/2006). Miltefosine (Impavido), was supplied by Zentaris GmbH (presently Aeterna Zentaris GmbH).
Authors' addresses: Anette Chrusciak-Talhari, Fundação de Medicina Tropical do Amazonas, Universidade Estadual do Amazonas, Manaus, AM, Brasil, E-mail: anettetalhari@terra.com.br. Reynaldo Dietze, Núcleo de Doenças Infecciosas, Universidade Federal do Espírito Santo, Vitória, ES, Brasil, E-mail: rdietze@ndi.ufes.br. Carolina Chrusciak Talhari, Fundação de Medicina Tropical do Amazonas, Manaus, AM, Brasil, E-mail: carolinatalhari@gmail.com. Roberto Moreira da Silva, Fundação de Medicina Tropical do Amazonas, Manaus, AM, Brasil, E-mail: robertomsjr@hotmail.com. Ellen Priscila Gadelha Yamashita, Fundação de Medicina Tropical do Amazonas, Manaus, AM, Brasil, E-mail: ellenpriscilla@ig.com.br. Gerson de Oliveira Penna, Núcleo de Medicina Tropical, Universidade de Brasilia, Brasilia, DF, Brasil, E-mails: gopenna@gmail.com or gpenna@saude.gov.br. Paulo Roberto Lima Machado, Serviço de Imunologia, Hosp. Univ. Prof. Edgar Santos, Universidade Federal da Bahia, Salvador, BA, Brasil, E-mail: prlmachado@uol.com.br. Sinésio Talhari, Fundação de Medicina Tropical do Amazonas, Manaus, AM, Brasil, E-mail: sinesiotalhari@terra.com.br.
References
- 1.Grimaldi G, Jr, Momen H, Naiff RD, McMahon-Pratt D. Characterization and classification of leishmanial parasites from humans, wild mammals, and sand flies in the Amazon region of Brazil. Am J Trop Med Hyg. 1991;44:645–661. doi: 10.4269/ajtmh.1991.44.645. [DOI] [PubMed] [Google Scholar]
- 2.Lainson R, Shaw JJ, Silveira FT, de Souza AA, Braga RR, Ishikawa EA. The dermal leishmaniases of Brazil, with special reference to the ecoepidemiology of the disease in Amazonia. Mem Inst Oswaldo Cruz. 1994;89:435–443. doi: 10.1590/s0074-02761994000300027. [DOI] [PubMed] [Google Scholar]
- 3.Ministério da Saúde, Fundação Nacional de Saúde, Centro Nacional de Epidemiologia. Manual de Vigilância da Leishmaniose Tegumentar Americana. Second edition. Brasília: Ministério da Saúde; 2007. http://portal.saude.gov.br/portal/arquivos/pdf/manual_lta_2ed.pdf (Brazil) Available at. Accessed May 11, 2010. [Google Scholar]
- 4.Sundar S, Jha TK, Thakur CP, Bhattacharya SK, Rai M. Oral miltefosine for the treatment of Indian visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2006;100((Suppl 1)):S26–S33. doi: 10.1016/j.trstmh.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Soto J, Berman J. Treatment of New World cutaneous leishmaniasis with miltefosine. Trans R Soc Trop Med Hyg. 2006;100((Suppl 1)):S34–40. doi: 10.1016/j.trstmh.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Soto J, Toledo J, Gutierrez P, Nicholls RS, Padilla J, Engel J, Fischer C, Voss A, Berman JD. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin Infect Dis. 2001;33:57–61. doi: 10.1086/322689. [DOI] [PubMed] [Google Scholar]
- 7.Soto J, Arana BA, Toledo J, Rizzo N, Vega JC, Diaz A, Luz M, Gutierrez P, Arboleda M, Berman JD, Junge K, Engel J, Sindermann H. Miltefosine for new world cutaneous leishmaniasis. Clin Infect Dis. 2004;38:1266–1272. doi: 10.1086/383321. [DOI] [PubMed] [Google Scholar]
- 8.Soto J, Toledo J, Valda L, Balderrama M, Rea I, Parra R, Ardiles J, Soto P, Gomez A, Molleda F, Fuentelsaz C, Anders G, Sindermann H, Engel J, Berman JD. Treatment of Bolivian mucosal leishmaniasis with miltefosine. Clin Infec Dis. 2007;44:350–356. doi: 10.1086/510588. [DOI] [PubMed] [Google Scholar]
- 9.Soto J, Rea J, Balderrama M, Toledo J, Soto P, Valda L, Berman JD. Efficacy of miltefosine for Bolivian cutaneous leishmaniasis. Am J Trop Med Hyg. 2008;78:210–211. [PubMed] [Google Scholar]
- 10.Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verástegui C, Lazo M, Loayza-Muro R, De Doncker S, Maurer A, Chappuis F, Dujardin JC, Llanos-Cuentas A. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis. 2007;195:1846–1851. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- 11.Llanos-Cuentas A, Tulliano G, Araujo-Castillo R, Miranda-Verastegui C, Santamaria-Castrellon G, Ramirez L, Lazo M, De Doncker S, Boelaert M, Robays J, Dujardin JC, Arevalo J, Chappuis F. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin Infect Dis. 2008;46:223–231. doi: 10.1086/524042. [DOI] [PubMed] [Google Scholar]
- 12.Saldanha AC, Romero GA, Merchan-Hamann E, Magalhães VA, Macedo VO. Comparative study between sodium stibogluconate BP 88 and meglumina antimoniate for cutaneous leishmaniasis treatment: I. Efficacy and safety. Rev Soc Bras Med Trop. 1999;32:383–387. doi: 10.1590/s0037-86821999000400008. [DOI] [PubMed] [Google Scholar]
- 13.Deps PD, Viana MC, Falqueto A, Dietze R. Evaluation of the efficacy and toxicity of N-methyl-glucamine vs. BP88 sodium stibogluconate in the treatment of localized cutaneous leishmaniasis. Rev Soc Bras Med Trop. 2000;33:535–543. doi: 10.1590/s0037-86822000000600004. [DOI] [PubMed] [Google Scholar]
- 14.Romero GA, Guerra MV, Paes MG, Macedo VO. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. Am J Trop Med Hyg. 2001;65:456–465. doi: 10.4269/ajtmh.2001.65.456. [DOI] [PubMed] [Google Scholar]
- 15.Garcia L, Kindt A, Bermudez H, Llanos-Cuentas A, De Doncker S, Arevalo J, Tintaya KW, Dujardin JC. Culture-independent species typing of neotropical Leishmania for clinical validation of a PCR-based assay targeting heat shock protein 70 genes. J Clin Microbiol. 2004;42:2294–2297. doi: 10.1128/JCM.42.5.2294-2297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berman J. Current treatment approaches to leishmaniasis. Curr Opin Infect Dis. 2003;16:397–401. doi: 10.1097/00001432-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Rotureau B, Ravel C, Nacher M, Couppie P, Curtet I, Dedet JP, Carme B. Molecular epidemiology of Leishmania (Viannia) guyanensis in French Guiana. J Clin Microbiol. 2006;44:468–473. doi: 10.1128/JCM.44.2.468-473.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero GA, Ishikawa E, Cupolillo E, Toaldo CB, Guerra MV, Paes MG, Macedo VO, Shaw JJ. Identification of antigenically distinct populations of Leishmania (Viannia) guyanensis from Manaus, Brazil, using monoclonal antibodies. Acta Trop. 2002;82:25–29. doi: 10.1016/s0001-706x(02)00002-5. [DOI] [PubMed] [Google Scholar]
- 19.McMahon-Pratt D, Bennett E, David JR. Monoclonal antibodies that distinguish subspecies of Leishmania braziliensis. J Immunol. 1982;129:926–927. [PubMed] [Google Scholar]


