Abstract
The objective of this study was to evaluate the behavior of different tests used for the diagnosis of visceral leishmaniasis (VL) in asymptomatic subjects living in an endemic area. No gold standard is available for the diagnosis of asymptomatic infection with Leishmania. In continuation of a previous study, 1,017 subjects living in a VL-endemic area were clinically reevaluated. Of these, 576 had at least one positive serological test in a first assessment. About 3 years after the first evaluation, none of the subjects had progressed to clinical VL. Among this group, 246 subjects were selected, and five serological tests (enzyme-linked immunosorbent assay p [ELISAp], ELISArK39, ELISArK26, indirect immunofluorescence test [IIFT] using L. amazonensis promastigote antigen, and an immunochromatographic test using rK39 antigen [TRALd]) and the Montenegro skin test (MST) were repeated. There was a significant increase in the number of subjects who tested positive in the MST, IIFT, ELISAp, and ELISArK39 in the second evaluation. For all tests, there were subjects who tested positive in the first evaluation and negative in the second evaluation. A positive result in the serological tests and MST in subjects from the endemic area studied did not indicate a risk of progression to VL and may only be temporary.
Introduction
In Brazil, the incidence of visceral leishmaniasis (VL) has increased over the years despite the control measures established by the Ministry of Health. These measures are mainly based on the identification and early treatment of human cases of the disease, serological surveys for canine leishmaniasis followed by euthanasia of infected dogs, and vector control (chemical and environmental management).1
The role of asymptomatic humans infected with Leishmania chagasi in the transmission chain of American VL has not been well-established, a fact that might explain why the control of the disease is not effective. Classically, humans are not considered to be a reservoir of L. chagasi in the New World,2,3 but this possibility could be properly evaluated only if a feasible and unequivocal tool for the diagnosis of asymptomatic cases were available. In addition, the identification of new infected individuals in endemic areas would also serve as an indicator of the effectiveness of control measures designed to reduce the number of individuals exposed to Leishmania. However, the diagnosis of asymptomatic infection with L. chagasi is still a matter of controversy and requires larger studies.4
The standardization and validation of diagnostic methods for VL usually include patients with the classical clinical presentation of the disease and controls. The gold-standard techniques used for the detection of the parasite are not justified in asymptomatic cases of the disease, because they may pose a risk or excessive discomfort to the individual without providing evident benefit. Thus, indirect methods for the evaluation of exposure to the parasite have been widely used as tools for the diagnosis of subclinical infection.
As early as in 1964, Manson-Bahr and Southgate5 called attention to a group of subjects with a positive skin test and no clinical history of leishmaniasis that did not develop the disease. Since then, the Montenegro skin test (MST) has become a diagnostic tool for previous infection with L. chagasi in VL-endemic areas and can be used to characterize asymptomatic infection according to various investigators.6–8
Serological tests also frequently identify individuals who live in endemic areas and have a positive VL test but show no clinical signs or symptoms of the disease. These individuals are classified as asymptomatic carriers of L. chagasi by different investigators. Several tests have been used to estimate the frequency of subclinical infection in these areas, with enzyme-linked immunosorbent assay (ELISA) using promastigote antigen being the most cited method.9–11 Burns and others,12 standardizing recombinant antigen rK39, suggested that ELISA using rK39 may serve as a tool for the diagnosis of asymptomatic cases.
In most studies regarding the diagnosis of asymptomatic cases of VL, the authors selected one serological test and/or skin test, and positive subjects were considered to be carriers of infection. However, comparison of five distinct serological tests performed at the same time showed low agreement between the results obtained.13 This panorama indicates the difficulty in selecting the best test for the detection of subclinical infection. Within this context, the present study evaluated the clinical and evolutionary behavior of a positive result in different VL serological tests and the MST in subjects from an endemic area.
Subjects and Methods
First, 1,241 subjects living in the endemic area of Porteirinha underwent clinical and laboratory evaluation between 1998 and 1999 using five serological tests (ELISA using promastigote antigen [ELISAp], ELISA using recombinant K39 [ELISArK39], ELISA using K26 [ELISArK26] antigens, an indirect immunofluorescence test using L. amazonensis promastigote antigen [IIFT], and an immunochromatographic test using rK39 antigen [TRALd])13 and the MST.14 None of these subjects had a history of a diagnosis of VL or had received specific treatment. In continuation of this cross-sectional study, the same population was reassessed between January and August 2002, about 3–4 years after the first evaluation.
All 1,241 subjects of the initial study were invited by letter to attend the Health Service for clinical reevaluation. However, we encountered difficulties in the delivery of the letter, because there were errors in the address provided or the subject had moved to another town. In addition, not all subjects who received the letter appeared at the service. Therefore, a total of 1,017 subjects (82% of the initial sample) were clinically reassessed by anamnesis and physical examination performed by an infectologist.
Among these subjects, 246 were selected, and a new blood sample was collected. All initial serological tests and the skin test were repeated based on the following information: group I (N = 122) consisted of subjects who, in the initial study, tested positive for leishmaniasis in the IIFT and/or TRALd and/or MST and negative for Chagas disease by indirect hemagglutination and IIFT, and group II (N = 124) consisted of subjects who tested negative in all five serological tests for leishmaniasis and in the MST and who presented negative serology for Chagas disease. Group II was matched for gender and age to group I. Group I should include all subjects of the initial study who met the inclusion criteria established. However, some of the subjects of this group refused to participate, and difficulties in delivering the invitation letter were encountered. Therefore, 122 of 195 subjects invited were available for reassessment and were matched for gender and age to group II. There were no significant differences in the previous results between the group invited and the group studied eventually.
The serum samples were collected between June and October 2002 and were processed in November and December 2002. The TRALd, ELISArK39, and ELISArK26 tests were performed at the Laboratory of Immunology, Federal University of Triângulo Mineiro (UFTM). Another aliquot stored at −20°C was kept on dry ice and sent to the Laboratory of Leishmaniasis and Vaccines, Institute of Biological Sciences, Federal University of Minas Gerais (ICB/UFMG), Belo Horizonte, for ELISA and IIFT using promastigote antigen. All laboratory researchers were unaware of the origin of the samples. All serum samples were analyzed by ELISAp, ELISArK26, ELISArK39, IIFT, and TRALd using the procedures of the initial study as described below.
TRALd (rK39).
For the immunochromatographic test, a kit (InBios International, Seattle, WA) consisting of paper strips coated with rK39 antigen was used according to manufacturer's instructions. The strips were stored at room temperature (28–30°C) or were refrigerated (2–8°C) and protected from humidity. The buffer solution was stored at 2–8°C. Readings were obtained 10 minutes later according to manufacturer's recommendations, and the results were reported as positive or negative.
IIFT.
The IIFT was performed as described by Camargo15 using as antigen L. amazonensis (MHOM/BR/60/BH6) promastigotes in the exponential growth phase cultured in liver infusion tryptose (LIT) medium. Fluorescein isothiocyanate-labeled human immunoglobulin G (IgG) antiglobulin obtained from rabbit immune serum was used as conjugate (Biomanguinhos, Rio de Janeiro, Brazil). All samples testing positive at a dilution of 1:80 were considered to be reactive.
MST.
Antigen of L. amazonensis promastigotes (IFLA/BR/67/PH8), produced at the Laboratory of Leishmaniasis and Vaccines, ICB/UFMG, was used at a standard concentration of 40 g/mL protein nitrogen diluted 1:10,000 in saline merthiolate.16 Antigen (0.1 mL) was applied intradermally to the anterior side of the left forearm. Readings were obtained 48–72 hours after injection, and the size of the papule was delimited with a ball-point pen.17 The reactions were considered to be positive when the mean of cross-sectional and longitudinal diameters was 5 mm or higher.
ELISA using recombinant antigens (ELISArK39 and ELISArK26).
The assays were carried out according to Badaró and others18 using recombinant K39 and K26 antigens of L. chagasi produced by InBios International. The serum samples were diluted 1:50 and 1:100 and developed with peroxidase-conjugated protein A (Sigma Co., St. Louis, MO).
ELISA using promastigote antigen (ELISAp).
The assay was carried out as described by Hommel and others19 and Voller and others20 using as antigen L. amazonensis (MHOM/BR/60/BH6) promastigotes in the stationary growth phase cultured in LIT medium and lysed by sonication. Serum samples were tested at an initial dilution of 1:80.
A standard permitting comparison between the first and second evaluation was established to define the ELISA cutoff values. Because the tests using sera from subjects of the non-endemic area (Argentina) were not repeated, this group did not exist as a negative control for calculation of the cutoff. We, therefore, chose as a reference subjects from an endemic area who could serve as controls. The TRALd, IIFT, and MST, whose cutoff values are well-defined in the literature, were used as a parameter (TRALd: presence of a line of the labeled antigen, IIFT: ≥ 1:80, and MST: ≥ 5 mm), and positive and negative results in the first and second evaluation could, therefore, be determined directly. In addition, the IIFT and TRALd presented the best sensitivity (> 80%) in the initial study. Thus, for ELISA, the cutoff in the two evaluations was established based on the results of the three previous tests (IIFT, TRALd, and MST) as follows: subjects with a positive IIFT and/or TRALd and/or MST in the first and second evaluation were defined as positive, and subjects with a negative result in the three tests of the two evaluations were defined as negative. Next, the receiver operating characteristic (ROC) curve provided by the MedCalc 10.1 program (MedCalc Software, Mariakerke, Belgium) was used to determine the cutoff values of each ELISA assay based on the calculation of sensitivity, specificity, and likelihood ratios.
A positive result in each diagnostic test was compared between the first and second evaluation by the McNemar test. Optical densities were compared between the two groups by the Mann-Whitney test. Agreement between the diagnostic tests was determined by the κ coefficient. Statistical analysis was performed using the Statistica 8.0 software (Statsoft, Inc., Tulsa, OK). A P value < 0.05 was considered to be significant.
Informed consent was obtained from all participants, and the project was approved by the Board of UFTM (Resolution 196/96 of the National Research Council, Brazilian Ministry of Health).21
Results
Clinical reassessment of the 1,017 subjects showed that none of them presented a clinical history or physical signs compatible with VL (fever, paleness, visceromegaly, adynamia, or weight loss) during the interval between the first and second evaluation or at the time of the second evaluation. It should be mentioned that 19 cases of human VL occurred in Porteirinha, the study area, during the period investigated. All signs and symptoms reported by the 246 subjects were compared with the results of the laboratory tests, and no association was observed between positive tests and the presence of any symptom.
The ELISA cutoff values were established by the ROC curve, considering the optical density at which the tests presented, at the same time, the best sensitivity, specificity, and likelihood ratios (Table 1). This definition was used to evaluate the evolution of the serological tests over time in the 246 subjects reevaluated (Table 2). For the MST, IIFT ELISAp, and ELISArK39, a significant increase in the number of seropositive subjects was observed in the second evaluation. In contrast, a significant reduction in the number of positive subjects was observed for the TRALd and ELISArK26 when comparing the two assessments. For all tests, there were subjects who tested negative in a previously positive test.
Table 1.
Cutoff values established for the ELISA assays based on the results of the ROC curve
| Diagnostic test | Cutoff value | TP | FP | FN | TN | Sensitivity (%) | Specificity (%) | LR+ | LR− | Area under the ROC curve (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| First evaluation | |||||||||||
| ELISAp | 0.070 | 83 | 27 | 39 | 97 | 68.0 | 78.2 | 3.1 | 0.4 | 0.779 (0.722–0.829) | < 0.001 |
| ELISArk39 | 0.308 | 72 | 33 | 50 | 91 | 59.0 | 73.4 | 2.2 | 0.6 | 0.667 (0.604–0.726) | < 0.001 |
| ELISArk26 | 0.084 | 100 | 91 | 22 | 33 | 81.9 | 26.6 | 1.1 | 0.7 | 0.511 (0.446–0.575) | 0.773 |
| Second evaluation | |||||||||||
| ELISAp | 0.150 | 110 | 32 | 46 | 58 | 70.5 | 64.4 | 2.0 | 0.5 | 0.697 (0.636–0.754) | < 0.001 |
| ELISArk39 | 0.323 | 102 | 46 | 54 | 44 | 65.4 | 48.9 | 1.3 | 0.7 | 0.580 (0.516–0.642) | 0.031 |
| ELISArk26 | 0.343 | 92 | 41 | 64 | 49 | 58.9 | 54.4 | 1.3 | 0.8 | 0.547 (0.483–0.610) | 0.213 |
TP = true positive; FP = false positive; FN = false negative; TN = true negative; LR+ = positive likelihood ratio; LR− = negative likelihood ratio; 95% CI = 95% confidence interval.
Table 2.
Comparison of the results of the diagnostic tests between the first and second evaluation of subjects from a VL-endemic area
| Diagnostic test | First evaluation | Second evaluation | ||
|---|---|---|---|---|
| Total positive | Remained positive | Became positive | Total positive | |
| Positive | 122/246 (49.6%) | 105/122 (86.1%) | 51/124 (41.1%) | 156/246 (63.4%) |
| MST | 71/246 (28.9%) | 62/71 (87.3%) | 52/175 (29.7%) | 144/246 (46.3%) |
| TRALd | 31/246 (12.6%) | 3/31 (9.7%) | 2/215 (0.9%) | 5/246 (2.0%) |
| IIFT | 31/246 (12.6%) | 24/31 (77.4%) | 63/215 (29.3%) | 87/246 (35.4%) |
| ELISAp | 110/246 (44.7%) | 84/110 (76.4%) | 58/136 (42.7%) | 142/246 (57.7%) |
| ELISArk39 | 105/246 (42.7%) | 75/105 (71.4%) | 73/141 (51.8%) | 148/246 (60.2%) |
| ELISArk26 | 191/246 (77.6%) | 107/191 (56.0%) | 26/55 (47.3%) | 133/246 (54.1%) |
| Negative | 124/246 (50.4%) | 73/124 (58.9%) | 17/122 (13.9%) | 90/246 (36.6%) |
| MST | 175/246 (71.1%) | 123/175 (70.3%) | 9/71 (12.7%) | 132/246 (53.7%) |
| TRALd | 215/246 (87.4%) | 213/215 (99.1%) | 28/31 (90.3%) | 241/246 (98.0%) |
| IIFT | 215/246 (87.4%) | 152/215 (70.7%) | 7/31 (22.6%) | 159/246 (64.6%) |
| ELISAp | 136/246 (55.3%) | 78/136 (57.4%) | 26/110 (23.6%) | 104/246 (42.3%) |
| ELISArk39 | 141/246 (57.3%) | 68/141 (48.2%) | 30/105 (28.6%) | 98/246 (39.8%) |
| ELISArk26 | 55/246 (22.4%) | 29/55 (52.7%) | 84/191 (44.0%) | 113/246 (45.9%) |
McNemar test (P < 0.001 for all comparisons between first and second evaluation).
The optical densities obtained with the three ELISA assays were significantly higher in the second evaluation than in the first one (Figure 1). This was even the case for ELISArK26, for which no increase in the number of seropositive subjects was observed.
Figure 1.
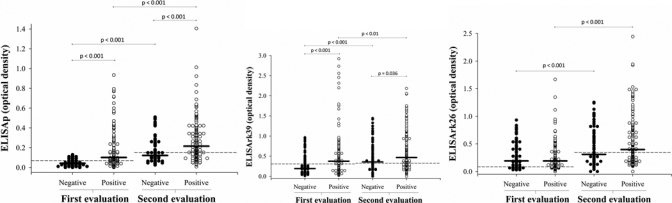
Comparison of optical densities obtained with the ELISAp, ELISArK39, and ELISArK26 tests between the first and second evaluation (— = test cutoff; – = group mean).
Regarding the agreement between the results of the tests in the second evaluation, the best κ coefficients (0.42) were observed for the comparison of IIFT/ELISAp (Table 3). The TRALd results presented low agreement with ELISArK39 (κ = 0.00).
Table 3.
κ coefficients obtained for the different diagnostic tests in the second evaluation of subjects from a VL-endemic area
| Comparisons | Agreement | κ (95% CI) | ||
|---|---|---|---|---|
| Second evaluation | Positive in both | Negative in both | Total agreement | |
| MST | ||||
| TRALd | 1 (0.9%) | 128 (97.0%) | 129 (52.4%) | −0.02 (−0.16–0.11) |
| IIFT | 46 (40.4%) | 91 (68.9%) | 137 (55.7%) | 0.09 (−0.03–0.22) |
| ELISAp | 74 (64.9%) | 64 (48.5%) | 138 (56.1%) | 0.13 (0.01–0.25) |
| ELISArk39 | 80 (70.2%) | 64 (48.5%) | 144 (58.5%) | 0.18 (0.06–0.30) |
| ELISArk26 | 69 (60.5%) | 68 (51.5%) | 137 (55.7%) | 0.12 (0.00–0.24) |
| TRALd | ||||
| IIFT | 4 (80.0%) | 158 (65.6%) | 162 (65.9%) | 0.05 (−0.11–0.21) |
| ELISAp | 3 (60.0%) | 102 (42.3%) | 105 (42.7%) | 0.00 (−0.11–0.11) |
| ELISArk39 | 3 (60.0%) | 96 (39.8%) | 99 (40.2%) | 0.00 (−0.10–0.10) |
| ELISArk26 | 1 (20.0%) | 109 (45.2%) | 110 (44.7%) | −0.03 (−0.14–0.09) |
| IIFT | ||||
| ELISAp | 77 (88.5%) | 94 (59.1%) | 171 (69.5%) | 0.42 (0.31–0.53) |
| ELISArk39 | 57 (65.5%) | 68 (42.8%) | 125 (50.8%) | 0.07 (−0.05–0.19) |
| ELISArk26 | 53 (60.9%) | 79 (49.7%) | 132 (53.7%) | 0.09 (−0.03–0.22) |
Discussion
In the initial study, a positive result in at least one of the serological tests studied was observed in 576 (46.4%) subjects and in the skin test in 146 (11.7%) subjects.13,14 Clinical reassessment 3–4 years after the first evaluation revealed that none of the subjects who tested positive developed classical VL. This result would be expected for subjects with a positive MST. However, for the serological tests, we observed that the disease was not diagnosed during the incubation period on any occasion, as shown by other investigators in Brazil for ELISAp.9,10 In India, follow-up of asymptomatic individuals (i.e., household contact with patients with a recent diagnosis of the disease) who tested positive by ELISArK39 showed that 69% of them developed kala-azar within a period of 12 months.22 Our results agree with other investigators who clinically followed up asymptomatic individuals in Brazil who tested seropositive by ELISArK39, and we found that none of them developed the disease.4,23,24
One aspect to be considered is the observation that, in the first study, the tests presented a specificity ranging from 96% to 100% using subjects from a non-endemic area as a reference. As a consequence, there was a false-positive rate of up to 4%. Similarly, part of the seropositive subjects followed-up in the endemic area may have had a false-positive test result.
Another limitation is that the 246 samples were retested 3–4 years after the first evaluation. Changes in the environmental conditions may have occurred during this period, which would interfere with the results. However, to test all samples at the same time, we would have to modify the storage conditions of the samples, with the initial samples being tested after 3–4 years of freezing, resulting in another type of bias.
The TRALd results need to be analyzed separately. In contrast to the other tests studied, the number of positive reactions was significantly smaller in the present investigation. This finding suggests differences in the interpretation of the test results in terms of their definition as positive or negative. The result of the TRALd depends on the visual evaluation of the presence or absence of a line labeled with rK39 on the immunochromatographic strip. In classical cases of the disease, this line is clearly visible and forms rapidly, with an unquestionable positive result in most cases. However, in asymptomatic individuals from endemic areas, this line is tenuous, and its interpretation may generate controversies. A lower quantity of antibodies in these subjects with asymptomatic infection may explain the lighter impression on the immunochromatographic strip. This observation was also made by Moreno and others,25 who established, for the diagnosis of asymptomatic infection by TRALd, that the test result should interpreted by three observers. The authors found wide disagreement between assessments and low reproducibility of the results (κ = 0.14).25 This bias might explain the low agreement between the TRALd and ELISArK39 results, because both tests use the same antigen. In the present study, we observed the best κ agreement between the results of tests that use similar antigens.13 Taken together, this rapid immunochromatographic test shows poor suitability for the diagnosis of asymptomatic L. chagasi infection.
The higher positivity rate obtained for the MST, IIFT, ELISAp, and ELISArK39 in the second evaluation might be associated with the permanence of the subject in the endemic area; the subject consequently continues to be exposed to the vector and thus, presents a chance of being infected within the interval of 3–4 years between assessments. An interesting finding was the increase of optical densities in the ELISA tests even in those subjects who continued to be positive. Repeated reinfections possibly exert a booster effect on the production of antileishmania antibodies, a fact that might explain the evident increase of optical density in the second evaluation.
With respect to a more detailed discussion of the MST results, the positivity rate obtained for the test increased from the first to the second evaluation, and as expected, most patients who tested positive in the first evaluation continued to be positive in the second. This result might be explained by possible new infections during the interval between the first and second evaluation. In addition, serial tests are known to trigger positivity in the subsequent test merely because of a booster effect of leishmanin.14,26 In contrast, one interesting finding was that nine previously positive subjects tested negative in the second evaluation. The MST is a delayed hypersensitivity reaction that depends on the cellular immune response to Leishmania antigens. Investigation of the subjects with a negative test result showed no immunosuppressive condition that would explain this behavior of the MST in any of them. Another possibility to explain this finding is a poor antigenicity of the antigen used in the second MST, as reported by other investigators.27 However, this explanation does not apply, because an increase in the frequency of positivity was observed in the present evaluation.
Some investigators believe that a positive MST may indicate immunity of the individual against the disease.5,6 In this respect, Bern and others27 showed that subjects from an endemic area with a positive MST presented a significantly lower chance to progress to classical VL than those with a negative MST. However, other investigators propose that, if this resistance really exists, it should be temporary, a suggestion that would explain the findings of the present study.28–30 In treated cases of the disease that progress to clinical cure, the MST classically becomes positive as a consequence of the restored immune response, which, in principle, should protect the individual against new relapses.31–33 However, some investigators have shown that this rate of post-clinical cure positivity is not as high as expected (about 40%) and may not increase over time post-treatment; additionally, negativity is not associated with a higher chance of relapse.27,32,34 These findings might be caused by the lack of a standard antigen used for the skin tests in different studies.
Difficulties exist in establishing a gold standard for the diagnosis of asymptomatic infection with L. chagasi.4,13,25 The comparison of tests may provide some additional information that contributes to the selection of the serological tests that best discriminates between infected and uninfected subjects. In this respect, ELISA assays, which are easily performed compared with IIFT and MST, would be an interesting option for this diagnosis. However, in the present study, none of tests presented a concordant positive result with the other tests or was superior for the identification of positive subjects. For confirmation of the results, it would be interesting to test the samples two times. This was not done because of the high cost of the large number of samples and number of tests evaluated. The ideal situation would be if only one test able to diagnose subclinical infection could be selected. It does not seem justifiable to routinely perform various tests only for detection of infection.
The low agreement between the results of the serological tests may indicate that each test detects different stages of infection in the group of asymptomatic subjects from the endemic area. On the basis of this assumption, all seropositive subjects would be carriers of asymptomatic infection, but the fact that none of the subjects progressed to clinical disease weakens this hypothesis. However, a low reproducibility of the serological tests used for the diagnosis of asymptomatic infection in the endemic area seems to be more likely. Therefore, the serological tests used here do not seem to be adequate tools for the diagnosis of asymptomatic infection.
Finally, the present study permits us to conclude that a positive result in the serological tests and in the MST in subjects from the endemic area studied did not indicate a risk of progression to VL and may only be temporary. Further studies are necessary to identify the best tool for the diagnosis of asymptomatic infection with L. chagasi in endemic areas and based on a more precise diagnosis of infected individuals, to define their true role in the maintenance of VL in urban areas.
ACKNOWLEDGMENTS
The authors thank Dr. Antônio Campos-Neto for providing the rK39 and rK26 antigens, Prof. Roberto Badaró for providing the TRALd strips, and Prof. Wilson Mayrink for the MST antigen.
Footnotes
Financial support: The study was supported by the National Council for Scientific and Technological Development and the National Health Foundation.
Authors' addresses: Luciana Almeida Silva, Héctor Dardo Romero, Gabriel Antônio Nogueira Nascentes, Virmondes Rodrigues, and Aluízio Prata, Department of Tropical Medicine and Infectology, Federal University of Triângulo Mineiro, Uberaba, Brazil, E-mails: lalmeidas@dcm.uftm.edu.br, hdardo_r_f@hotmail.com, gabrielnog@yahoo.com.br, vrodrigues@mednet.com.br, and a_prata@mednet.com.br.
Reprint requests: Luciana Almeida Silva, Department of Tropical Medicine and Infectology, Federal University of Triângulo Mineiro, Brazil, Caixa Postal: 118, CEP 38001-970, Uberaba, MG, Brazil, E-mail: lalmeidas@dcm.uftm.edu.br.
References
- 1.Brasil Ministério da Saúde, Fundação Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica . Manual de vigilância e controle da leishmaniose visceral. Brasil: Ministério da Saúde; 2006. pp. 1–122. [Google Scholar]
- 2.Deane MP, Deane LM. Observações sobre a transmissão da leishmaniose visceral no Ceará. Hospital. 1955;48:347–364. [Google Scholar]
- 3.Costa CHN, Gomes RBB, Silva MRB, Garcez LM, Ramos PKS, Santos RS, Shaw JJ, David JR, Maguire JH. Competence of human host as a reservoir for Leishmania chagasi. J Infect Dis. 2000;182:997–1000. doi: 10.1086/315795. [DOI] [PubMed] [Google Scholar]
- 4.Moreno EC, Gonçalves AV, Chaves AV, Melo MN, Lambertucci JR, Andrade ASR, Corrêa DN, Antunes CMF, Carneiro M. Inaccuracy of enzyme-linked immunosorbent assay using soluble and recombinant antigens to detect asymptomatic infection by Leishmania infantum. PLoS Negl Trop Dis. 2009;5:e536. doi: 10.1371/journal.pntd.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manson-Bahr PEC, Southgate BA. Recent research on Kala Azar in East Africa. J Trop Med Hyg. 1964;67:79–84. [PubMed] [Google Scholar]
- 6.Shido SA, Akuffo HO, Mohamed AA, Huldt G, Nilsson LA, Ouchterlony O, Thorstensson R. Visceral leishmaniasis in Somalia: prevalence of leishmanin-positive and seropositive inhabitants in an endemic area. Trans R Soc Trop Med Hyg. 1995;89:21–24. doi: 10.1016/0035-9203(95)90640-1. [DOI] [PubMed] [Google Scholar]
- 7.Cabello PH, Lima AMVM, Azevedo ES, Krieger H. Familial aggregation of Leishmania chagasi infection in northeastern Brazil. Am J Trop Med Hyg. 1995;53:364–365. doi: 10.4269/ajtmh.1995.52.364. [DOI] [PubMed] [Google Scholar]
- 8.D'Oliveira A, Jr, Costa SRM, Barbosa AB, Orge MGO, Carvalho EM. Asymptomatic Leishmania chagasi infection in relatives and neighbors of patients with visceral leishmaniasis. Mem Inst Oswaldo Cruz. 1997;92:15–20. doi: 10.1590/s0074-02761997000100003. [DOI] [PubMed] [Google Scholar]
- 9.Badaró R, Jones TC, Carvalho EM, Sampaio DP, Reed SG, Barral A, Teixeira R, Johnson WD., Jr New perspectives on a sub clinical form of visceral leishmaniasis. J Infect Dis. 1986;154:1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- 10.Evans TG, Teixeira MJ, Mcauliffe IT, Vasconcelos IAB, Vasconcelos AW, Sousa AQ, Lima JWO, Pearson RD. Epidemiology of visceral leishmaniasis in northeast Brazil. J Infect Dis. 1992;166:1124–1132. doi: 10.1093/infdis/166.5.1124. [DOI] [PubMed] [Google Scholar]
- 11.Jerônimo SMB, Teixeira MJ, Sousa AQ, Thielking P, Pearson RD, Evans TG. Natural history of Leishmania (Leishmania) chagasi infection in northeastern Brazil: long-term follow-up. Clin Infect Dis. 2000;30:608–609. doi: 10.1086/313697. [DOI] [PubMed] [Google Scholar]
- 12.Burns JM, Jr, Shreffler WG, Benson DR, Ghalib HW, Badaró R, Reed SG. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci USA. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero HD, Silva LA, Silva-Vergara ML, Rodrigues V, Costa RT, Carvalho SFG, Alecrim W, Moraes-Souza H, Prata A. Comparative study of serologic tests for the diagnosis of asymptomatic visceral leishmaniasis in an endemic area. Am J Trop Med Hyg. 2009;81:27–33. [PubMed] [Google Scholar]
- 14.Borges VC, Ruiz MCM, Gomes PM, Colombo AR, Silva LA, Romero HD, Prata A. Intradermorreação de Montenegro após sucessivas repetições do teste em Porteirinha, MG. Rev Soc Bras Med Trop. 2003;36:249–251. doi: 10.1590/s0037-86822003000200009. [DOI] [PubMed] [Google Scholar]
- 15.Camargo ME. Fluorescent antibody test for the serodiagnosis of American trypanosomiasis. Technical modification employing preserved culture forms of Trypanosoma cruzi in a slide test. Rev Inst Med Trop Sao Paulo. 1966;8:227–234. [PubMed] [Google Scholar]
- 16.Melo MN, Mayrink W, Costa CA, Magalhães PA, Dias M, Williams P, Araújo FG, Coelho MV, Batista SM. Padronização do antígeno de Montenegro. Rev Inst Med Trop Sao Paulo. 1977;19:161–164. [PubMed] [Google Scholar]
- 17.Sokal JE. Editorial: measurement of delayed skin-test response. N Engl J Med. 1975;293:501–502. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 18.Badaró R, Reed SG, Barral A, Orge MGO, Jones TC. Evaluation of micro enzyme-linked immunosorbent assay (ELISA) for antibodies in American visceral leishmaniasis: antigen selection for detection of infection-specific response. Am J Trop Med Hyg. 1986;35:72–78. doi: 10.4269/ajtmh.1986.35.72. [DOI] [PubMed] [Google Scholar]
- 19.Hommel M, Peters W, Ranque J, Quilici M, Lanotte G. The micro-ELISA technique in the serodiagnosis of visceral leishmaniasis. Ann Trop Med Parasitol. 1978;72:213–218. doi: 10.1080/00034983.1978.11719308. [DOI] [PubMed] [Google Scholar]
- 20.Voller A, Bidwell DE, Bartlett A. Enzyme immunoassay in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53:55–65. [PMC free article] [PubMed] [Google Scholar]
- 21.Brazil Ministry of Health . National Research Council. Resolution 196/96. Brazil: Ministry of Health; 1996. [Google Scholar]
- 22.Singh S, Kumari V, Singh N. Predicting kala-azar disease manifestation in asymptomatic patients with latent Leishmania donovani infection by detection of antibody against recombinant k39 antigen. Clin Diagn Lab Immunol. 2002;9:568–572. doi: 10.1128/CDLI.9.3.568-572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nascimento MDSB, Neto APBN, Silva NL, Coaracy GAV, Souza EC, Silva MH, Lindoso E, Sousa WB, Magalhães CA, Brito GO, Almeida PEA, Bezerra GFB, Viana GMC. Progressão da intradermorreação de Montenegro e a sororreatividade por ELISA com os antígenos rk39 e crude em indivíduos assintomáticos de área endêmica de leishmaniose visceral. Rev Soc Bras Med Trop. 2000;33((Suppl 1)):41. [Google Scholar]
- 24.Viana GMC, Nascimento MDSB, Viana MGC, Burattini MN.2000Avaliação prospectiva do antígeno rK39 como indicador de doença ativa em leishmaniose visceral Rev Soc Bras Med Trop 33(Suppl 1)319–320.10967602 [Google Scholar]
- 25.Moreno EC, Melo MN, Lambertucci JR, Serufo JC, Andrade ASR, Antunes CMF, Genaro O, Carneiro M. Diagnosing human asymptomatic visceral leishmaniasis in an urban area of the State of Minas Gerais, using serological and molecular biology techniques. Rev Soc Bras Med Trop. 2006;39:421–427. doi: 10.1590/s0037-86822006000500001. [DOI] [PubMed] [Google Scholar]
- 26.De Luca PM, Mayrink W, Santiago MA, Nogueira R, Conceição-Silva F, Melo G, Mendonça SC. Randomized, double-blind, placebo-controlled study on the immunogenicity of the leishmanin skin test. Trans R Soc Trop Med Hyg. 2003;97:709–712. doi: 10.1016/s0035-9203(03)80109-8. [DOI] [PubMed] [Google Scholar]
- 27.Bern C, Amann J, Haque R, Chowdhury AM, Kurkjian KM, Vaz L, Wagatsuma Y, Breiman RF, Secro WE, Maguire JH. Loss of leishmanin skin test antigen sensitivity and potency in a longitudinal study of visceral leishmaniasis in Bangladesh. Am J Trop Med Hyg. 2006;75:744–748. [PubMed] [Google Scholar]
- 28.Southgate BA, Oriedo VEB. Studies in the epidemiology of East African Leishmaniasis. 3. Immunity as a determinant of geographical distribution. J Trop Med Hyg. 1967;70:1–4. [PubMed] [Google Scholar]
- 29.Southgate BA, Manson-Bahr PEC. Studies in the epidemiology of East African Leishmaniasis. 4. The significance of the positive leishmanin test. J Trop Med Hyg. 1967;70:29–33. [PubMed] [Google Scholar]
- 30.Levy L, Mendes E. Impaired cell-mediated immunity in patients with Kala-azar. Allergol Immunopathol (Madr) 1981;9:109–112. [PubMed] [Google Scholar]
- 31.Pampiglione S, Manson-Bahr PE, La Placa M, Borgatti MA, Musumeci S. Studies in Mediterranean leishmaniasis. 3. The leishmanin skin test in kala-azar. Trans R Soc Trop Med Hyg. 1975;69:60–68. doi: 10.1016/0035-9203(75)90012-7. [DOI] [PubMed] [Google Scholar]
- 32.Silva LA, Romero HD, Prata A, Costa RT, Nascimento E, Carvalho SFG, Rodrigues V. Immunologic tests in patients after clinical cure of visceral leishmaniasis. Am J Trop Med Hyg. 2006;75:739–743. [PubMed] [Google Scholar]
- 33.Neogy AB, Nandy A, Dastidar BG, Chowdhury AB. Leishmanin skin test in Indian kala-azar. Trans R Soc Trop Med Hyg. 1986;80:454–455. doi: 10.1016/0035-9203(86)90341-x. [DOI] [PubMed] [Google Scholar]
- 34.Gidwani K, Rai M, Chakravarty J, Boelaert M, Sundar S. Short report: evaluation of leishmanin skin test in Indian visceral leishmaniasis. Am J Trop Med Hyg. 2009;80:566–567. [PubMed] [Google Scholar]


