Abstract
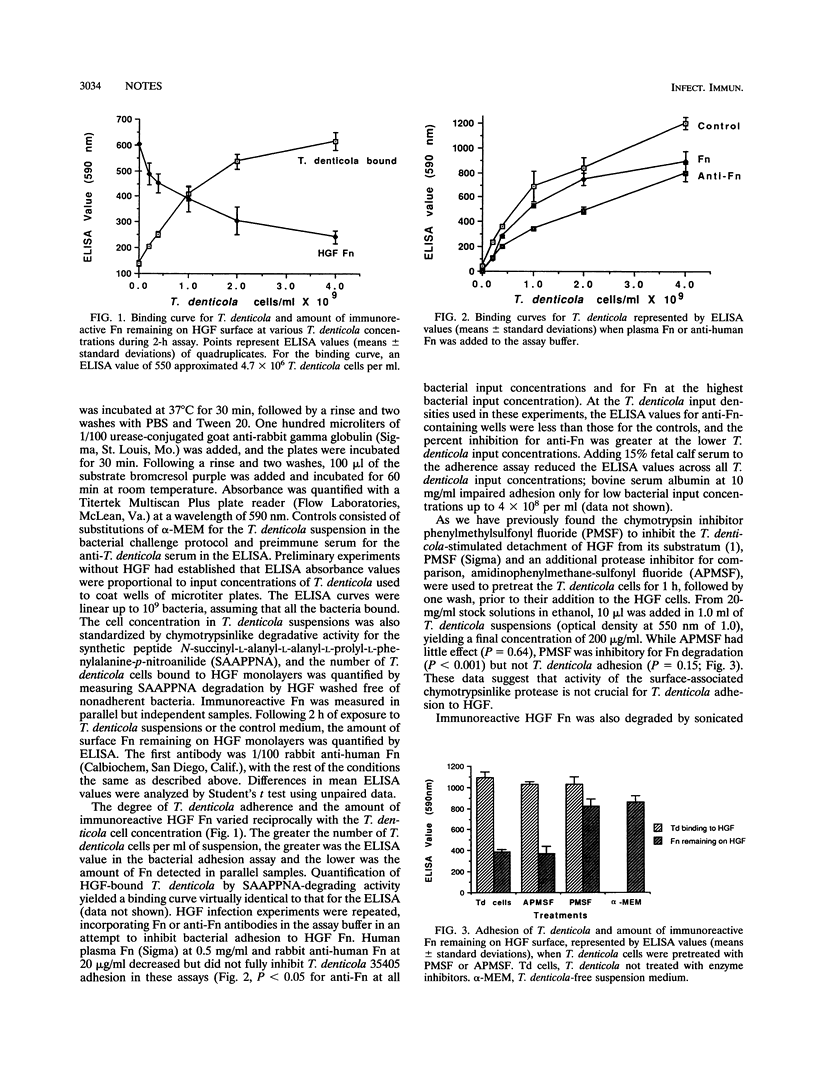
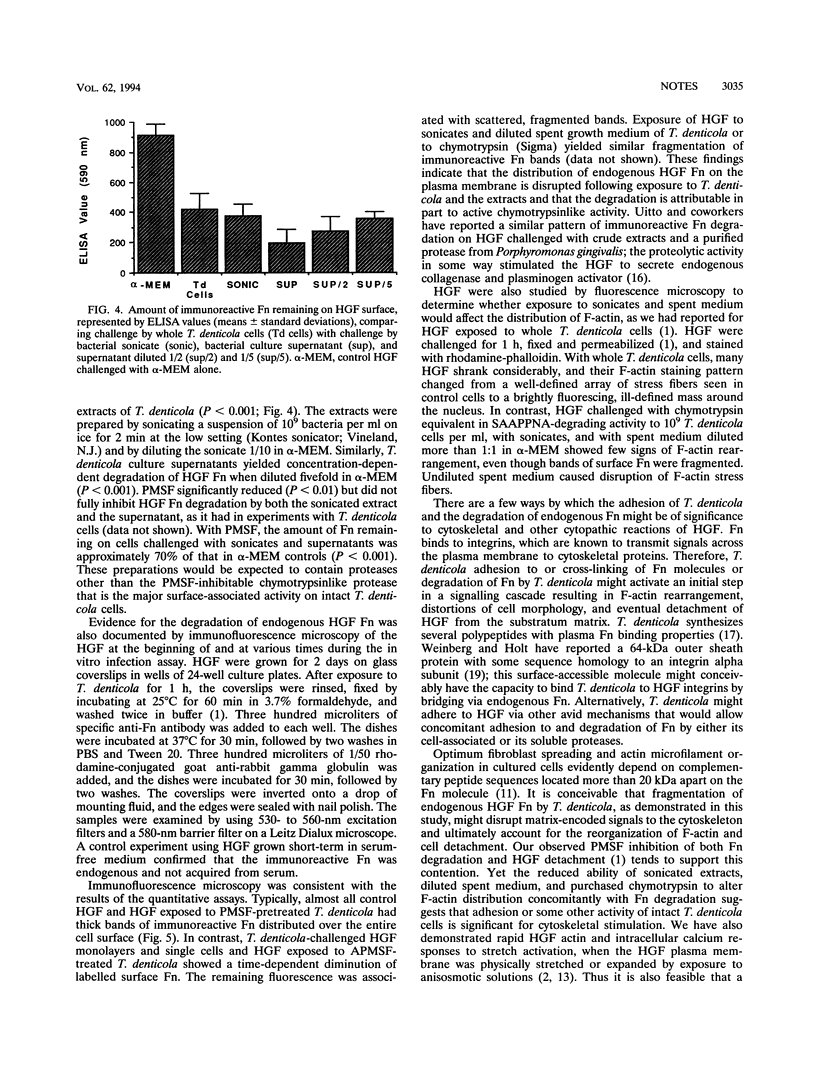
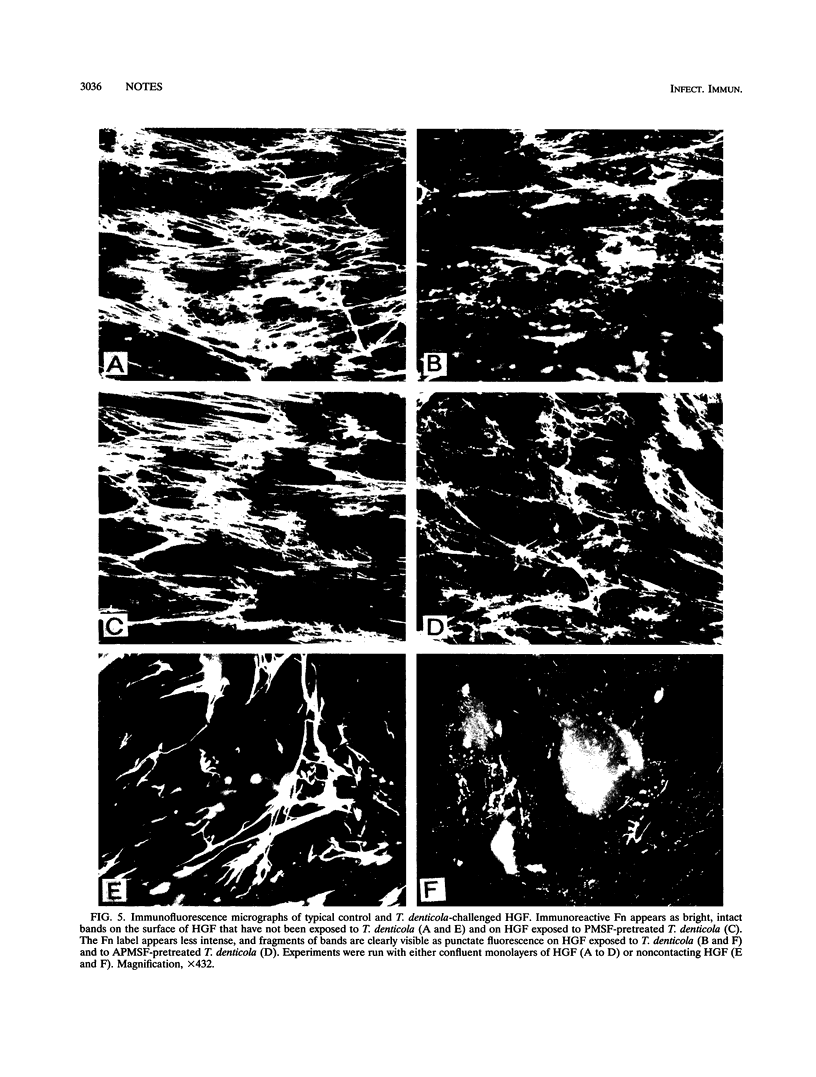
Treponema denticola adhesion and degradation of fibronectin (Fn) on human gingival fibroblasts (HGF) were studied by immunofluorescence and enzyme-linked immunosorbent assays. The number of adherent bacteria increased and the amount of immunoreactive Fn decreased as a function of increasing T. denticola concentration. The distribution of cell-bound Fn was punctate in micrographs. Anti-human Fn impaired bacterial adhesion to HGF. Phenylmethylsulfonyl fluoride inhibited Fn degradation but not adhesion. Sonicated extracts and diluted spent growth medium degraded HGF Fn but, unlike intact T. denticola cells, they hardly stimulated F-actin rearrangements.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehni P. C., Song M., McCulloch C. A., Ellen R. P. Treponema denticola induces actin rearrangement and detachment of human gingival fibroblasts. Infect Immun. 1992 Aug;60(8):3360–3368. doi: 10.1128/iai.60.8.3360-3368.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer H., Taichman N. S., Shenker B. J. Suppression of fibroblast proliferation by oral spirochetes. Infect Immun. 1984 Jul;45(1):155–159. doi: 10.1128/iai.45.1.155-159.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J. R., Ellen R. P. Clustering of fibronectin adhesins toward Treponema denticola tips upon contact with immobilized fibronectin. Infect Immun. 1994 Jun;62(6):2214–2221. doi: 10.1128/iai.62.6.2214-2221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J. R., Ellen R. P. Tip-oriented adherence of Treponema denticola to fibronectin. Infect Immun. 1990 Dec;58(12):3924–3928. doi: 10.1128/iai.58.12.3924-3928.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Uitto V. J., McBride B. C. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect Immun. 1990 Feb;58(2):347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo M., Singh U., McBride B. C., Uitto V. J. Sulfhydryl-dependent attachment of Treponema denticola to laminin and other proteins. Infect Immun. 1991 Nov;59(11):4230–4237. doi: 10.1128/iai.59.11.4230-4237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. The role of spirochetes in periodontal disease. Adv Dent Res. 1988 Nov;2(2):275–283. doi: 10.1177/08959374880020021201. [DOI] [PubMed] [Google Scholar]
- Mikx F. H., Jacobs F., Satumalay C. Cell-bound peptidase activities of Treponema denticola ATCC 33520 in continuous culture. J Gen Microbiol. 1992 Sep;138(9):1837–1842. doi: 10.1099/00221287-138-9-1837. [DOI] [PubMed] [Google Scholar]
- Obara M., Kang M. S., Yamada K. M. Site-directed mutagenesis of the cell-binding domain of human fibronectin: separable, synergistic sites mediate adhesive function. Cell. 1988 May 20;53(4):649–657. doi: 10.1016/0092-8674(88)90580-6. [DOI] [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender N., McCulloch C. A. Quantitation of actin polymerization in two human fibroblast sub-types responding to mechanical stretching. J Cell Sci. 1991 Sep;100(Pt 1):187–193. doi: 10.1242/jcs.100.1.187. [DOI] [PubMed] [Google Scholar]
- Riviere G. R., Weisz K. S., Simonson L. G., Lukehart S. A. Pathogen-related spirochetes identified within gingival tissue from patients with acute necrotizing ulcerative gingivitis. Infect Immun. 1991 Aug;59(8):2653–2657. doi: 10.1128/iai.59.8.2653-2657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto V. J., Grenier D., Chan E. C., McBride B. C. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988 Oct;56(10):2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto V. J., Larjava H., Heino J., Sorsa T. A protease of Bacteroides gingivalis degrades cell surface and matrix glycoproteins of cultured gingival fibroblasts and induces secretion of collagenase and plasminogen activator. Infect Immun. 1989 Jan;57(1):213–218. doi: 10.1128/iai.57.1.213-218.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T., Nakatani Y., Nakamura Y., Namikawa I. Fibronectin-binding proteins of a human oral spirochete Treponema denticola. Microbiol Immunol. 1993;37(1):75–78. doi: 10.1111/j.1348-0421.1993.tb03182.x. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Chemical and biological activities of a 64-kilodalton outer sheath protein from Treponema denticola strains. J Bacteriol. 1991 Nov;173(21):6935–6947. doi: 10.1128/jb.173.21.6935-6947.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect Immun. 1990 Jun;58(6):1720–1729. doi: 10.1128/iai.58.6.1720-1729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]