Abstract
Climatic factors influence the incidence of vector-borne diseases such as malaria. They modify the abundance of mosquito populations, the length of the extrinsic parasite cycle in the mosquito, the malarial dynamics, and the emergence of epidemics in areas of low endemicity. The objective of this study was to investigate temporal associations between weekly malaria incidence in 1,993 children < 15 years of age and weekly rainfall. A time series analysis was conducted by using cross-correlation function and autoregressive modeling. The regression model showed that the level of rainfall predicted the malaria incidence after a time lag of 9 weeks (mean = 60 days) and after a time lag between one and two weeks. The analyses provide evidence that high-resolution precipitation data can directly predict malaria incidence in a highly endemic area. Such models might enable the development of early warning systems and support intervention measures.
Introduction
Malaria is the most common vector-borne infectious disease in the world, with nearly 250 million estimated clinical cases among 3.3 billion persons at risk in 2008 and approximately 1 million deaths each year.1 With a vast majority of cases (85%) Sub-Saharan Africa carries most of the burden.1,2 In malaria-endemic areas, children < 5 years of age are at highest risk for malaria morbidity and mortality. The number of disability-adjusted life years, a measure of disease burden caused by malaria, was estimated to be 34 million for 2004 worldwide, with 31 million in sub-Saharan Africa.3 Malaria alone costs Africa's economy more than US$ 12 billion annually.4 In the Ashanti Region of Ghana, malaria is prevalent during the entire year and one of the major in-patient causes of death.5
In contrast to a retrogressive trend of malaria morbidity and mortality in some areas, malaria burden has been increasing in many other areas because of factors such as deteriorating health systems, growing drug and insecticide resistance, failure of water management, and climate, socioeconomic, sociodemographic, and land-use factors.1,6,7 Simple methods that enable accurate forecasting, early warning, and timely case detection in low- and high-transmission areas are needed to enable implementation of more effective control measures.8,9
Climate and meteorologic factors (precipitation, temperature, and relative humidity) have considerable impact on Anopheles vector abundance and the extrinsic cycles that the parasites perform inside mosquitoes. Thus, they may affect malaria incidence and constitute driving forces of malaria epidemics.10–12 Therefore, precipitation, which is probably the most important climatic factor in tropical areas with relatively constant temperature and humidity, was the focus of our models.
Our objective was to investigate the association between weekly malaria incidence in children < 15 years of age and rainfall in two village clusters of high endemicity during an 18-month period (end of May 2007 to the end of November 2008) to assess the extent to which precipitation data can be used to predict malaria incidence in a holoendemic area.
Materials and Methods
Study area.
This hospital-based survey was conducted at the Child Welfare Clinic and the Pediatric Ward of the Agogo Presbyterian Hospital, Asante Akim North District, Ashanti Region, Ghana (Figure 1). The district lies within the moist semi-deciduous forest belt, although there are some transitional zones caused by farming and logging activities. The climate is tropical and has a mean annual ambient temperature of 26°C and two rainy seasons; the first occurs during May–July and the second occurs during September–with monthly rainfall up to rainfall in ≤ 400 mm. The dry season or the harmattan (a dry and dusty West African trade wind from the arid and dessert areas north of Ghana) occurs during December–April and is associated with drought conditions. The topography of the study district is generally undulating and the altitude variation is approximately 600 meters between the lowest area near the Volta Lake (152 meters) and the Akwapim-Mampong range (≤ 762 meters). The local economy is mainly agriculture; major staple food crops include maize, cassava, plantain, cocoyam, and yam.13
Figure 1.
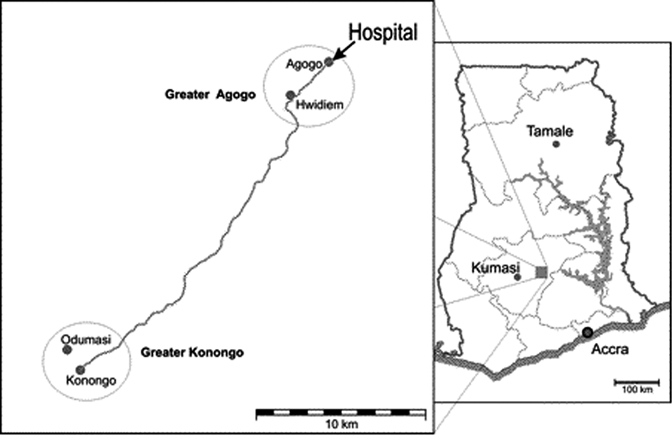
Map of the two village clusters Greater Agogo (Agogo and Hwidiem) and Greater Konongo (Konongo and Odumasi), Asante Akim North District, Ashanti Region, central Ghana. Circles indicate the two village clusters, and the solid line indicates mains roads. There are additional settlements along the main road.
The main malaria vectors are mosquitoes of the Anopheles gambiae complex and An. funestus. Malaria is holoendemic in this area, Plasmodium falciparum accounts for most (> 90%) human malaria infections.14
In this study, two village clusters of four villages were included: two (Agogo and Hwidiem) in Greater Agogo and two other adjacent villages (Konongo and Odumasi) in Greater Konongo (Figure 1). The two areas are approximately 20 km apart and are connected by a main road.
The population figures according to the 2004 census were 13,559 and 1,402 for Agogo and Hwidiem, respectively, and 15,383 and 8,502 for Konongo and Odumasi, respectively. Greater Agogo has an area of 16 km2 and an altitude of 430 meters above sea level. Greater Konongo has an area of 18 km2 and an altitude of 230 meters above sea level.
Data collection and analysis.
All hospital visits of children < 15 years of age from the two village clusters were included. Criteria were an axillary temperature ≥ 37.5°C and a positive result for a P. falciparum parasitemia (> 0 parasites/μL) during the study period of 90 weeks (end of May 2007 to the end of November 2008). Parasite examinations were conducted according to quality-controlled standardized procedures described elsewhere.15 Children with cases of malaria who visited the hospital within 21 days after the initial malaria diagnosis were considered as relapses and were not included as a new case. The study was reviewed and approved by the Committee on Human Research, Publications, and Ethnics, School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
For the calculation of cumulative incidences, population size, admission rate, proportion recruited, and proportion of the population seeking health care in the study hospital were considered. We used census data to determine that 42% of the population was < 15 years of age.16 According to a community survey conducted in 2007, 93% of persons from Greater Agogo and 25% of persons from Greater Konongo were seeking health care at the Agogo Presbyterian Hospital. The denominator/reference population for the calculation of incidences was corrected for these proportions. Likewise, the reference population was corrected for the proportion of children that met the inclusion criteria but were not recruited (30%). Weekly malaria incidences per 1,000 inhabitants < 15 years of age were then calculated for each village cluster.
Data on daily rainfall in Agogo and Konongo during March 2007–November 2008 were obtained from the Ghana Meteorological Agency (Accra, Ghana). For both areas, weekly precipitation were calculated.
To model the association between rainfall and malaria incidence during March 2007–November 2008 in the two clusters by linear regression analysis, we used the logarithm of the weekly incidence. If the number of weekly malaria cases equaled zero, we assumed the logarithm of half of the minimum weekly incidence excluding zero. The cross-correlation function between the time series of the weekly precipitation and the log-transformed weekly incidence was analyzed to assess time lags with peak correlations between the course of malaria incidence and the course of precipitation. These time lags were used in the linear regression of precipitation on log-transformed malaria incidence. Furthermore, to account for autoregression of the incidence time series, autoregressive terms of white noise had to be included in the regression model. The following general regression model results were used:
where It = incidence, Rt = precipitation and et = white noise (e∼N(0,1)) at time t, μ = geometric mean of weekly incidence, l(i) = ith lag (i = 1,…,k), and αi (i = 1,…,k) and βi (i = 1,…,m), respectively, being regression coefficients.
The regression models were applied to estimate expected incidence. Furthermore, using the estimated and observed malaria incidence, we determined that the amount of explained variance (R2) could provide a measure of overall goodness-of-fit. STATA/SE software version 10 (StataCorp LP, College Station, TX) was used for calculations.
Results
During the study period, 7,313 hospital visits by children < 15 years were reported: 5,276 cases from Greater Agogo and 2,037 cases from Greater Konongo. A total of 1,993 (27%) fulfilled the case definition for malaria and thus were included in the analysis. The annual incidence was 270.6 and 144.2 per 1,000 per year in Greater Agogo and Greater Konongo, respectively. The weekly incidence per 1000 inhabitants and weekly precipitation varied over time in both village clusters (Table 1 and Figure 2).
Table 1.
Population, malaria incidence, and precipitation in the two village clusters, Ghana
| Characteristic | Greater Agogo | Greater Konongo |
|---|---|---|
| Total population | 14,961 | 23,885 |
| Children < 15 years of age | 5140 | 2008 |
| No. of malaria cases* | 1610 | 383 |
| Total yearly incidences† | 270.6 | 144.2 |
| Minimum weekly incidences‡ | 1.0 | 0 |
| Maximum weekly incidences‡ | 12.4 | 7.3 |
| Minimum weekly precipitation§ | 0 | 0 |
| Maximum weekly precipitation§ | 20.3 | 30.2 |
No. of cases over the study interval of 90 weeks (end of May 2007–end of November 2008).
Incidences per year and 1,000 inhabitants.
Incidences per week and 1,000 inhabitants.
Mean weekly precipitation in millimeters.
Figure 2.
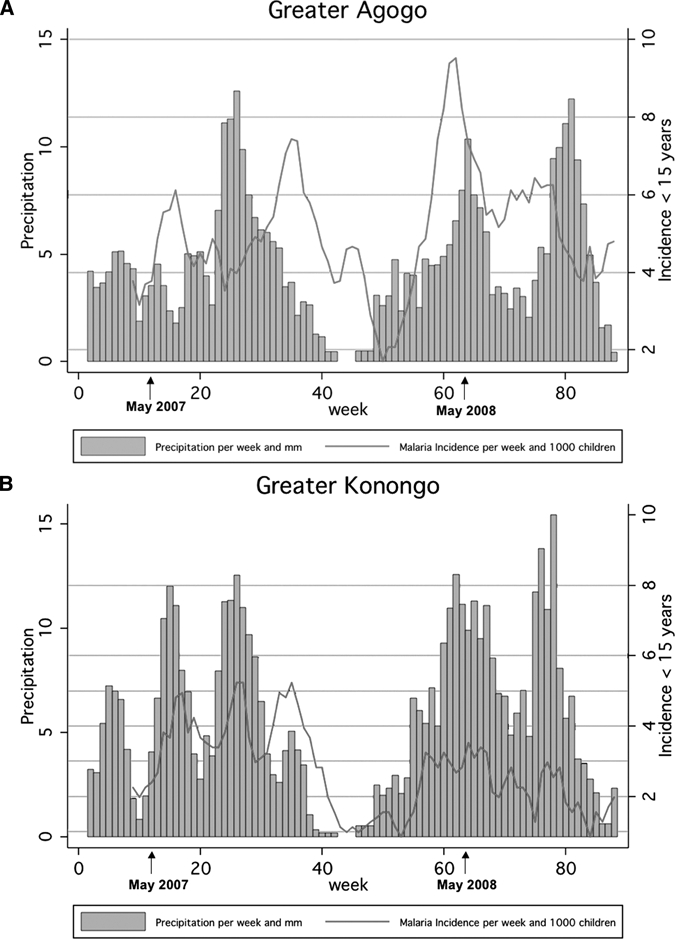
Weekly precipitation and four-week average of malaria incidences per week and 1,000 children < 15 years of age two village clusters, Ghana. A, Greater Agogo, B, Greater Konongo.
The weekly malaria incidence lagged a few weeks behind weekly precipitation (Figure 2). The cross-correlation functions for the two village clusters showed a seasonal pattern of the influence of precipitation on the log-transformed incidence (Figure 3). The cross-correlation function of Greater Agogo clearly indicated a 26-week cycle. Because of low case numbers, the cross-correlation function of Greater Konongo exhibited a large fluctuation with respect to a sinusoidal course. However, the phase difference, i.e., the time lag between a peak in precipitation and in malaria incidence was nine weeks for both areas. Additionally, peaks of the cross-correlations functions at lags of one week and two weeks in Greater Konongo and in Greater Agogo, respectively, indicated a relevant influence of preceding rainfall events on malaria incidence.
Figure 3.
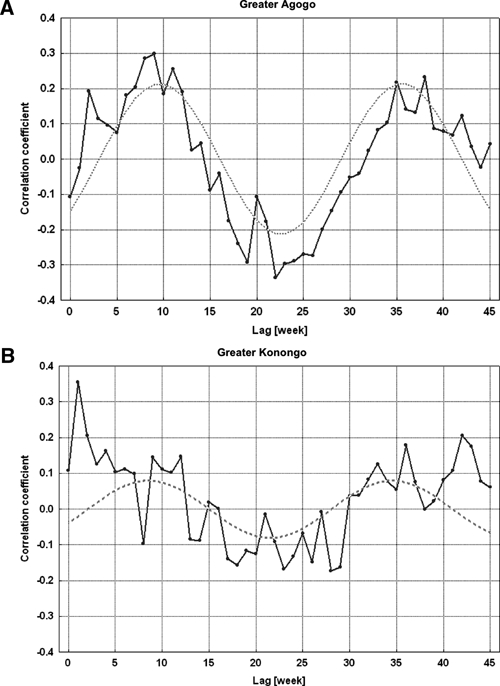
Cross-correlation between log-transformed malaria incidences of children < 15 years of age and precipitation in the two village clusters, Ghana. A, Greater Agogo. B, Greater Konongo. The dashed lines represent approximations of the cross-correlation functions by sinus functions with period length of a half year, i.e., 26 weeks.
If one considers the results of cross-correlation between precipitation and incidence, time lags of nine weeks for both village clusters and one and two weeks for Greater Konongo and Greater Agogo, respectively, were applied for regression modeling. A first-order autoregressive term for the white noise was sufficient to model the autocorrelation of the incidence. For the village cluster of Greater Agogo, all coefficients of the regression model were statistically significant, and the model could explain 63% of incidence variation (R2 = 0.634) (Table 2). Because of low case numbers in Greater Konongo, the regression model could explain only 31% of incidence variation (R2 = 0.311), but with a similar regression coefficient (Table 2). Observed and expected malaria incidences, including 95% confidence intervals, in children < 15 years of age according to regression modeling in the two village clusters are shown in Figure 4. The time series of expected malaria incidences, estimated by the regression models, clearly showed a time pattern that closely followed the time pattern of the observed incidences.
Table 2.
Estimated malaria model parameters, Ghana
| Model parameter* | Greater Agogo | Greater Konongo | ||||
|---|---|---|---|---|---|---|
| Coefficient | SE | P† | Coefficient | SE | P† | |
| Mean log incidence rate [μ]‡ | 1.366 | 0.097 | < 0.001 | 0.381 | 0.158 | 0.016 |
| lag: 1 week [α1]§ | – | – | – | 0.042 | 0.014 | 0.003 |
| Lag: 2 weeks [α1] § | 0.022 | 0.009 | 0.017 | – | – | – |
| Lag: 9 weeks [α2] § | 0.017 | 0.009 | 0.051 | 0.022 | 0.014 | 0.122 |
| White noise [β1] | 0.467 | 0.103 | < 0.001 | 0.262 | 0.112 | 0.019 |
| R2¶ | 0.634 | 0.311 | ||||
For explanation of model parameters, see text (model formula).
By t-test.
Unit = 10−3/week.
Unit = mm × 10−3/week.
Explained variance by regression model.
Figure 4.
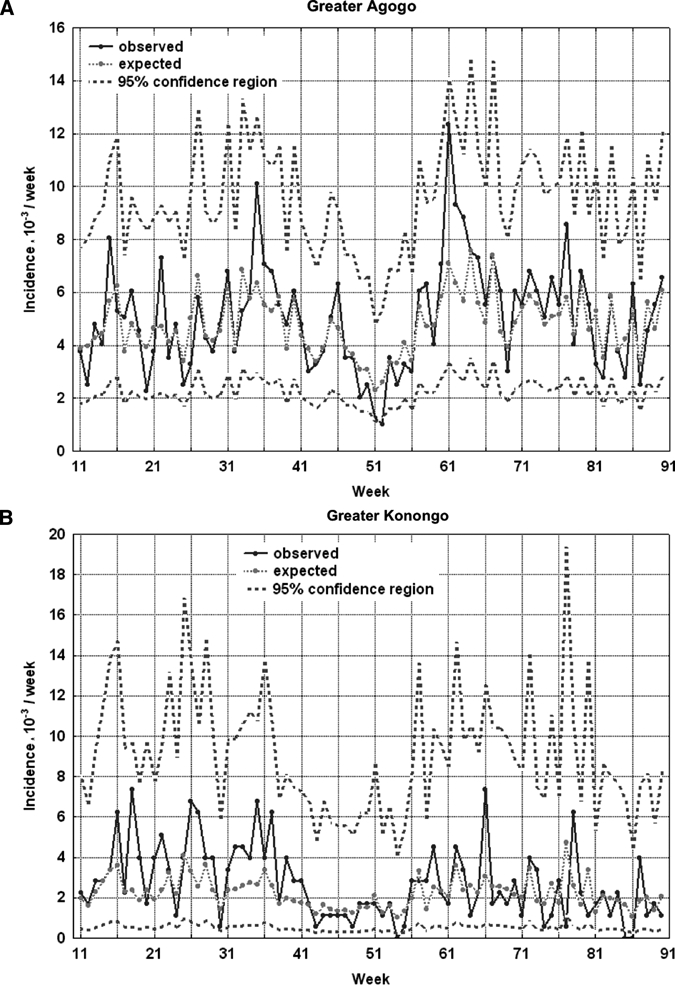
Weekly malaria incidence per 1,000 children < 15 years of age in the two village clusters, Ghana. A, Greater Agogo. B, Greater Konongo. Observed incidence (continuous line) and expected incidence by means of regression modeling with precipitation as predictor (dashed line) with 95% confidence interval (broad dashed lines).
Discussion
The analysis of the malaria epidemiology in two village clusters in Ghana with high endemicity indicated a strong temporal association between rainfall and incidence of malaria. The cross-correlation functions gave the most appropriate congruence of malaria incidence and precipitation with a time lag of 9 weeks (mean = 60 days). This period coincides with the theoretical vector-parasite-host cycle of the three organisms involved under optimum conditions, assuming that the first blood meal of Anopheles is on an infected human and that the temperature is at mean ≥ 25°C (Figure 5). This cycle has three components: 1) the growth of the Anopheles vector from egg to adults that are able to transmit parasites; 2) the development of the Plasmodium parasite in the vector from gametocytes to sporozoites that are able to infect humans; and 3) the incubation period in the human host from infection to the onset of malarial symptoms.17,18 According to this timeline, an incidence peak can be expected between day 50 and day 60 after breeding (Figure 5).
Figure 5.
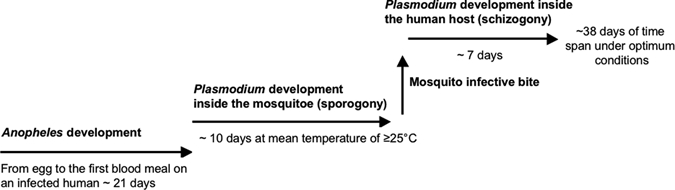
Model of time required from precipitation and deposition of mosquito eggs to onset of malarial symptoms in the human host under optimal conditions. In our study site in Ghana, Anopheles development needs approximately 19 days. Two days after hatching, the female Anopheles mosquitoes need their first blood meal and after uptake of gametocytes, the development in the mosquito (sporogony) takes a minimum of 10 days. After transmission of sporozoites during a bite by an infective mosquito, Plasmodium development in the human host (schizogony) takes approximately seven days. Therefore, it takes a minimum of approximately 38 days under optimum conditions from precipitation and deposition of mosquito eggs to the outcome malaria (assuming that the first blood meal of Anopheles is on an infected human and that the mean temperature is ≥ 25°C). During the rainy season more breeding habitats are available. This factor increases the likelihood that more mosquitoes hatch in a certain period of time and that they reach successively higher densities. The life expectancy of An. funestus and An. gambiae is approximately 30 days at a mean temperature of ≥ 25°C.18 After the first two days until a female mosquito needs its first blood meal plus the 10 additional days of sporogony and the 7 days of schizogony described in our model, the theoretically remaining life expectancy of a mosquito after the minimum time span of 38 days from precipitation to malaria is 11 more days (30 – 2 – 10 – 7 = 11). Therefore, the mosquito stays infective for 11 more days and can transmit the disease. Thus, if one considers our model, the highest Anopheles densities can be first expected after approximately 50 days (minimum time span of 38 days + 11 days of remaining life expectancy). The successively higher densities could theoretically then be highest after approximately 60 days, which complies with our results.
Additionally, the cross-correlation functions showed a strong association between rainfall and the malaria incidence one or two weeks later dependent on the village cluster. This shorter time lag might be caused by higher biting activities of adult mosquitoes at the beginning of rainy season and in due course breeding habitats for the mosquito that soon become available.19,20
The modulation of the estimated and observed incidence rates was less coherent in Greater Konongo than in Greater Agogo (demonstrated by a smaller R2 in Greater Konongo), which may be explained by the lower case numbers and therefore a decreased power. Amplitudes of the expected incidences were lower than those of the observed incidences because of the phenomenon of the regression to the mean.
The regression model was not able to predict a peak incidence in Greater Agogo in May 2008 over a time period of 4 weeks (Figure 2A). This observation may be explained by exceptional meteorologic conditions. To validate this possibility, temperature and relative humidity from the area was analyzed during March 2007–May 2008. The prevailing temperature in the study area during this period was in the optimum temperature range for An. gambiae and An. funestus, which is approximately ≥ 25°C to 30°C. Thus, temperature should not have influenced the abundance of mosquitoes. Relative humidity, which in Ghana is constantly 85–90% during the entire year, also did not show any aberrations during this interval. The malaria incidence peak in May 2008 was also found in other villages in our study area, which argues against temporal-spatial change of exposure. A temporal reporting bias is improbable because the number of all hospital admissions or the proportion of children included in the study did not increase during this period.
Although the reason for the short increase of the malaria incidence is unknown, the peak does not contradict the model. First, there are certainly temporal and spatial events that influence the malaria incidence, which are unpredictable in the model, e.g., temporal control measures or impassable roads for a limited time. Second, not all relevant events can be detected, e.g., short but intensive rainfall periods or fluctuations of population and hospital personnel. Third, the amount of rainfall per week might not provide all information necessary to predict the likelihood of mosquito breeding and survival. Thus, the optimal conditions for development of breeding sites might be determined by the amount of rainfall until a certain threshold. There is a minimum amount of rainfall required to maintain constant water bodies of a critical size and at other sites, heavy rainfall can have an opposite effect by rinsing out breeding sites.21 Such a putative threshold might have been achieved at the end of February 2008 when an extraordinary high amount of rainfall was recorded.
Other investigators have also reported a strong temporal link between climatic indices and increasing risk for malaria disease. In China, increasing monthly malaria incidences were positively correlated with monthly mean climatic variables (relative humidity, temperature, and precipitation), with a one-month lagged effect.22 In Eastern Sudan, rainfall was a significant climatic variable in the transmission of the disease.23 However, in a study conducted in central India, no relationship between rainfall and malaria incidence was observed.24 Instead, in other malaria-endemic areas, mean or minimum temperatures were the best predictors of clinical malaria.25,26 However, most such analyses have been carried out at monthly time scale and were not able to provide a time lag on a weekly scale. More precise results with a resolution of weeks such as this study are rarely reported.27
In addition to climatic factors, the risk for malaria transmission or mosquito abundance may be influenced by other factors such as seasonal fluctuations of migrant workers or the accessibility of the hospital in the rainy season when roads are flooded. However, the study area has a relative stable population, and considerable plantations that would attract seasonal field workers are not present. Additionally, the main road, along which the surveyed villages are located, is a well-constructed tarred road, which is passable in the rainy season. Therefore, seasonal variation in accessibility should not influence temporal changes of malaria incidence. The importance of socioeconomic factors such as ethnic group, parent's education and occupation, use of protective measures, and the family's financial situation on malaria transmission have been described in a number of studies.28–30 Geographic and environmental factors such as altitude and land cover have also been suggested as variables influencing the transmission of malaria. The abundance of water bodies and favorable temperatures, maize plantings, extensive deforestation, or farmland have been associated with increased larval or mosquito abundance and thus increased risk for malaria transmission in human populations.31–34 Other studies have used geographic information systems and satellite imagery to investigate environmental factors that potentially drive the dynamics of malaria vector populations34–36 and other vector-borne and zoonotic diseases such as dengue fever or hantavirus.37–39
It has been shown that the efficacy of control measures such as intermittent preventive treatment (IPT) can be strongly dependent on the present malaria incidence,40–42 and it can be assumed that direct and contextual effects increase with malaria risk after an intervention. The results of the present study highlight that it is feasible in holoendemic areas to predict fluctuations in the malaria incidence with information that is easy to obtain. This enables optimizing the planning of malaria interventions.
ACKNOWLEDGMENTS
We thank the interviewees for their participation in this study, the fieldworkers of the Kumasi Centre for Collaborative Research in Tropical Medicine for their assistance, members of the Public Health Unit of the Agogo Presbyterian Hospital for their enduring collaboration, and the Ghana Meteorological Agency for providing climate data. The work is part of the PhD thesis of Anne Caroline Krefis.
Footnotes
Financial support: The study was supported by a Swiss Foundation.
Authors' addresses: Anne Caroline Krefis, Norbert Georg Schwarz, Andreas Krüger, Julius Fobil, Wibke Loag, and Jürgen May, Bernhard-Nocht-Institute for Tropical Medicine, Infectious Disease Epidemiology, Hamburg, Germany, E-mails: krefis@bni-hamburg.de, schwarznorbert@bni-hamburg.de, krueger@bni-hamburg.de, fobil@bni-hamburg.de, loag@bni-hamburg.de, and may@bni-hamburg.de. Bernard Nkrumah, Samuel Acquah, and Nimako Sarpong, Kumasi Centre for Collaborative Research in Tropical Medicine, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, E-mails: skrakyo@yahoo.com, ekubanus2000@yahoo.co.uk, and nimakosarpong@yahoo.com. Yaw Adu-Sarkodie, Kwame Nkrumah University of Science and Technology, School of Medical Sciences, Kumasi, Ghana, E-mail: sax@ghanatel.com.gh. Ulrich Ranft, Environmental Health Research Institute, Heinrich Heine University of Düsseldorf, Düsseldorf, Germany, E-mail: ranft@uni-duesseldorf.de.
References
- 1.World Health Organization . World Malaria Report 2009. Geneva: World Health Organization; 2009. http://www.who.int/malaria/world_malaria_report_2009/en/index.html Available at. Accessed January 20, 2010. [Google Scholar]
- 2.Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, Snow RW. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;15:e1000290. doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Burden of Disease: DALYs. Geneva: World Health Organization; 2004. http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_part4.pdf Available at. Accessed June 17, 2009. [Google Scholar]
- 4.Roll Back Malaria, Global Malaria Partnership 2009. http://www.rollbackmalaria.org/keyfacts.html Available at. Accessed June 17, 2009.
- 5.De-Graft Aikins A. Ghana's neglected chronic disease epidemic: a developmental challenge. Ghana Med J. 2007;41:154–159. [PMC free article] [PubMed] [Google Scholar]
- 6.Nájera JA, Kouznetzsov RL, Delacollette C. Malaria Epidemics. Detection and Control, Forecasting and Prevention. Geneva: World Health Organization; 1998. http://www.emro.who.int/rbm/publications/epidemics_najera.PDF WHO/MAL/98.1084. Available at. Accessed June 17, 2009. [Google Scholar]
- 7.World Bank . Malaria at a Glance. World Bank Report. Washington, DC: World Bank; 2001. [Google Scholar]
- 8.World Health Organization . Malaria Epidemics: Forecasting, Prevention, Early Detection and Control. From Policy to Practice. Report of an Informal Consultation. Leysin, Switzerland: World Health Organization; 2003. http://www.searo.who.int/LinkFiles/Malaria_MalariaERBM2004.pdf December 8–10, 2003. Available at. Accessed June 17, 2009. [Google Scholar]
- 9.World Health Organization . Malaria Early Warning Systems: Concepts, Indicators and Partners: A Framework for Field Research in Africa. Geneva: World Health Organization; http://apps.who.int/malaria/cmc_upload/0/000/014/807/mews2.pdf Available at. Accessed June 17, 2009. [Google Scholar]
- 10.Gomez-Elipe A, Otero A, Van Herp M, Aguirre-Jaime A. Forecasting malaria incidences based on monthly case reports and environmental factors in Karuzi, Burundi, 1997–2003. Malar J. 2007;6:129. doi: 10.1186/1475-2875-6-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson MC, Mason SJ, Phindela T, Connor SJ. Use of rainfall and sea surface temperature monitoring for malaria early warning in Botswana. Am J Trop Med Hyg. 2005;73:214–221. [PubMed] [Google Scholar]
- 12.Thomson MC, Doblas-Reyes FJ, Mason SJ, Phindela T, Morse AP, Palmer TN. Malaria early warning based on seasonal climate forecasts from multimodel ensembles. Nature. 2006;439:576–579. doi: 10.1038/nature04503. [DOI] [PubMed] [Google Scholar]
- 13.Information about All Districts in Ghana. http://www.ghanadistricts.com/districts/?news&r=2&_=18 Available at. Accessed June 26, 2009.
- 14.Browne EN, Frimpong E, Sievertsen J, Hagen J, Hamelmann C, Dietz K, Horstmann RD, Burchard GD. Malariometric update for the rainforest and savanna of Ashanti region, Ghana. Ann Trop Med Parasitol. 2000;94:15–22. [PubMed] [Google Scholar]
- 15.Trape JF. Rapid evaluation of malaria parasite density and standardization of thick smear examination for epidemiological investigations. Trans R Soc Trop Med Hyg. 1985;79:181–184. doi: 10.1016/0035-9203(85)90329-3. [DOI] [PubMed] [Google Scholar]
- 16.Ghana Statistical Service. http://www.statsghana.gov.gh/surveys/CENSUS2000/survey0/index.html Available at. Accessed November 23, 2009.
- 17.Lucius R, Loos-Frank B. Biologie von Parasiten. 2nd edition. Berlin, Heidelberg: Springer Verlag; 2008. [Google Scholar]
- 18.Service M. Medical Entomology for Students. 4th edition. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 19.Rozendaal JA. Relations between Anopheles darlingi breeding habitats, rainfall, river level, and malaria transmission rates in the rain forest of Suriname. Med Vet Entomol. 1992;6:16–22. doi: 10.1111/j.1365-2915.1992.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 20.Ndiaye PI, Bicout DJ, Mondet B, Sabatier P. Rainfall triggered dynamics of Aedes mosquito aggressiveness. J Theor Biol. 2006;243:222–229. doi: 10.1016/j.jtbi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Gillies MT. In: Malaria: Principles and Practice of Malariology. Wernsdorfer WH, McGregor I, editors. Edinburgh: Churchill Livingstone; 1988. pp. 453–485. (Anopheline mosquitos: vector behaviour and bionomics). [Google Scholar]
- 22.Bi P, Tong S, Donald K, Parton KA, Ni J. Climatic variables and transmission of malaria: a 12-year data analysis in Shuchen county, China. Public Health Rep. 2003;118:65–71. doi: 10.1093/phr/118.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himeidan YE, Hamid EE, Thalib L, Elbashir MI, Adam I. Climatic variables and transmission of falciparum malaria in New Halfa, eastern Sudan. East Mediterr Health J. 2007;13:17–24. [PubMed] [Google Scholar]
- 24.Singh N, Sharma VP. Patterns of rainfall and malaria in Madhya Pradesh, central India. Ann Trop Med Parasitol. 2002;96:349–359. doi: 10.1179/000349802125001113. [DOI] [PubMed] [Google Scholar]
- 25.Yé Y, Louis VR, Simboro S, Sauerborn R. Effect of meteorological factors on clinical malaria risk among children: an assessment using village-based meteorological stations and community-based parasitological survey. BMC Public Health. 2007;7:101. doi: 10.1186/1471-2458-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abeku TA, Van Oortmarssen GJ, Borsboom G, De Vlas SJ, Habbema JD. Spatial and temporal variations of malaria epidemic risk in Ethiopia: factors involved and implications. Acta Trop. 2003;87:331–340. doi: 10.1016/s0001-706x(03)00123-2. [DOI] [PubMed] [Google Scholar]
- 27.Peterson I, Borrell LN, El-Sadr W, Teklehaimanot A. A temporal-spatial analysis of malaria transmission in Adama, Ethiopia. Am J Trop Med Hyg. 2009;81:944–949. doi: 10.4269/ajtmh.2009.08-0662. [DOI] [PubMed] [Google Scholar]
- 28.Kreuels B, Kobbe R, Adijei S, Kreuzberg C, Von Reden C, Bäter K, Klug S, Busch W, Adijei O, May J. Spatial variation of malaria incidences in young children from a geographically homogeneous area with high endemicity. J Infect Dis. 2008;197:85–93. doi: 10.1086/524066. [DOI] [PubMed] [Google Scholar]
- 29.Koram KA, Bennett S, Adiamah JH, Greenwood BM. Socio-economic risk factors for malaria in a peri-urban area of The Gambia. Trans R Soc Trop Med Hyg. 1995;89:146–150. doi: 10.1016/0035-9203(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 30.Krefis AC, Schwarz NG, Nkrumah B, Acquah S, Loag W, Sarpong N, Adu-Sarkodie Y, Ranft U, May J. Prinicpal component analysis of socioeconomic factors and association with malaria in children from the Ashanti region. Ghana. Malar J. 2010;13:201. doi: 10.1186/1475-2875-9-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye-Ebiyo Y, Pollack RJ, Spielman A. Enhanced development in nature of larval Anopheles arabiensis mosquitoes feeding on maize pollen. Am J Trop Med Hyg. 2000;63:90–93. doi: 10.4269/ajtmh.2000.63.90. [DOI] [PubMed] [Google Scholar]
- 32.Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Life-table analysis of Anopheles arabiensis in western Kenya highlands: effects of land covers on larval and adult survivorship. Am J Trop Med Hyg. 2007;77:660–666. [PubMed] [Google Scholar]
- 33.Munga S, Minakawa N, Zhou G, Mushinzimana E, Barrack OO, Githeko AK, Yan G. Association between land cover and habitat productivity of malaria vectors in western Kenyan highlands. Am J Trop Med Hyg. 2006;74:69–75. [PubMed] [Google Scholar]
- 34.Brooker S, Clarke S, Njagi JK, Polack S, Mugo B, Estambale B, Muchiri E, Magnussen P, Cox J. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop Med Int Health. 2004;9:757–766. doi: 10.1111/j.1365-3156.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 35.Rogers DJ, Randolph SE, Snow RW, Hay SI. Satellite imagery in the study and forecast of malaria. Nature. 2002;415:710–716. doi: 10.1038/415710a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts DR, Paris JF, Manguin S, Harbach RE, Woodruff R, Rejmankova E, Polanco J, Wullschleger B, Legters LJ. Predictions of malaria vector distribution in Belize based on multispectral satellite data. Am J Trop Med Hyg. 1996;54:304–308. doi: 10.4269/ajtmh.1996.54.304. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Bi P, Hiller JE. Climate change and the transmission of vector-borne disease: a review. Asia Pac J Public Health. 2008;20:64–76. doi: 10.1177/1010539507308385. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz AC, Ranft U, Piechotowski I, Childs JE, Brockmann SO. Risk factors for human infection with Puumala virus, southwestern Germany. Emerg Infect Dis. 2009;15:1032–1039. doi: 10.3201/eid1507.081413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ. 2000;78:1136–1147. [PMC free article] [PubMed] [Google Scholar]
- 40.Kobbe R, Kreuzberg C, Adjei S, Thompson B, Langefeld I, Thompson PA, Abruquah HH, Kreuels B, Ayim M, Busch W, Marks F, Amoah K, Opoku E, Meyer CG, Adjei O, May J. A randomized controlled trial of extended intermittent preventive antimalarial treatment in infants. Clin Infect Dis. 2007;45:16–25. doi: 10.1086/518575. [DOI] [PubMed] [Google Scholar]
- 41.Kobbe R, Adjei S, Kreuzberg C, Thompson B, Langefeld I, Thompson PA, Abruquah HH, Kreuels B, Ayim M, Busch W, Marks F, Amoah K, Opoku E, Meyer CG, Adjei O, May J. Malaria incidence and efficacy of intermittent preventive treatment in infants (IPTi) Malar J. 2007;6:163. doi: 10.1186/1475-2875-6-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sokhna C, Cissé B, Bâ el H, Milligan P, Hallett R, Sutherland C, Gaye O, Boulanger D, Simondon K, Simondon F, Targett G, Lines J, Greenwood B, Trape JF. A trial of the efficacy, safety and impact on drug resistance of four drug regimens for seasonal intermittent preventive treatment for malaria in Senegalese children. PLoS ONE. 2008;3:1471. doi: 10.1371/journal.pone.0001471. [DOI] [PMC free article] [PubMed] [Google Scholar]


