Abstract
We determined the Human Blood Index (HBI) of malaria mosquito vectors in Equatorial Guinea. We used a polymerase chain reaction (PCR)-based methodology to identify blood meal sources in engorged mosquitoes. We observed high HBI values, indicating that these vectors are highly anthropophilic despite intensive intradomicillary application of residual insecticides. Our results suggest that estimating the HBI can be a relatively simple and easy way to evaluate the efficacy of antimalaria interventions where an observed diversion to non-human hosts may successfully contribute to the interruption of malaria transmission.
The Human Blood Index (HBI) represents the proportion of blood meals derived from humans by mosquito vectors. It may be used to estimate the human biting habit, an important component of vectorial capacity, as a proxy measure of malaria transmission.1–3 Anopheles gambiae is widely regarded as a highly anthropophilic and domestic mosquito vector.4 However, some studies report An. gambiae s.s. preferentially feeding on dogs or cattle over human hosts.5–7 The zoophily of these vectors could be explained as a response to indoor residual spraying (IRS) interventions combined with the use of long-lasting insecticide-treated nets (LLINs).6 The insecticides used in such interventions have excito-repellent effects that drive host-seeking mosquitoes outdoors, where they may come into contact with non-human hosts.
In 2004, a comprehensive antimalaria intervention, led by Marathon Oil Corporation in close collaboration with the Equatoguinean Ministry of Health, was initiated to reduce the malaria burden on Bioko Island.8 Specific intervention activities included indoor application of residual insecticides, distribution of LLINs, and improved case management and detection of malarial illness. Routine entomological monitoring activities included measures of seasonal abundance, sporozoite rates, and frequency and distribution of insecticide-resistant alleles.9–11 In 2007, a similar antimalaria initiative funded by the United Nations Global Fund was expanded to include the mainland portion of Equatorial Guinea.8,12 Until now, no host selection studies have been conducted in the country, and the degree of anthropophily among malaria vectors is yet to be determined.
It may be that, as a consequence of indoor-based antivector interventions, host-seeking mosquitoes are diverted to seek blood meals in outdoor venues, where a greater diversity of non-human hosts may be encountered and fed on. Accordingly, we performed a blood meal analysis to assess the degree of anthropophily and determine the range of hosts. In particular, we estimated the proportion of mosquitoes that fed on human or other mammalian hosts by employing a polymerase chain reaction (PCR) assay to amplify mammalian mitochondrial DNA (mtDNA) from engorged mosquito vectors.
Collections took place on Bioko Island and 18 sites in mainland Equatorial Guinea (Table 1). The collection period spanned from March to September 2009. Ultraviolet (UV)-modified Centers for Disease Control and Prevention (CDC) light traps were used to sample indoor areas monthly from 6:00 pm to 6:00 am.13 Although this collection method is not optimal for this study, because indoor-collected mosquitoes are more likely to feed on humans resting indoors, collection of large numbers of mosquitoes outdoors is difficult because of the inconsistencies associated with outdoor collection schemes.14 To attempt to limit this inherent bias in our sampling scheme, we also collected a small subsample of mosquitoes from light traps placed outside of homes on Bioko Island. Similar outdoor collections were not available for analysis from the mainland sampling sites. Our approach was a necessary compromise to perform a comparison between indoor and outdoor feeding patterns.
Table 1.
Collection sites, number of mosquitoes per site, and map coordinates
| Collection site | Number of mosquitoes | Latitude (N) | Longitude (E) |
|---|---|---|---|
| Bioko Island | 45 | 03°29.256′ | 008°41.205′ |
| Yengue | 27 | 02°12.630′ | 009°52.489′ |
| Ayamiken | 81 | 02°06.663′ | 010°01.381′ |
| Micomeseng | 15 | 02°01.308′ | 010°37.880′ |
| Ebebiyin | 34 | 02°02.703′ | 011°18.757′ |
| Etofili | 14 | 01°52.186 | 009°46.812′ |
| Ukomba | 15 | 01°50.604′ | 009°44.730′ |
| Ngolo | 26 | 01°51.679′ | 009°47.441′ |
| Niefang | 73 | 01°50.504′ | 010°15.845′ |
| Bicurga | 21 | 01°34.818′ | 010°27.921′ |
| Anisok | 13 | 01°52.141′ | 010°45.007′ |
| Nsok Nsomo | 14 | 01°53.877′ | 011°05.799′ |
| Mongomo | 12 | 01°38.673′ | 011°14.760′ |
| Mbini | 26 | 01°36.449′ | 009°37.561′ |
| Cogo | 13 | 01°05.826′ | 009°43.745′ |
| Acurenam | 17 | 01°02.819′ | 010°37.900′ |
| Aconibe | 7 | 01°16.979′ | 010°54.072′ |
| Nsork | 15 | 01°08.348′ | 011°16.131′ |
| Evinayong | 13 | 01°20.973′ | 010°34.627′ |
After collection, mosquitoes were placed in tubes containing 80% ethanol for transport. DNA from the mosquito abdomen was extracted using the QIAGEN DNeasy Blood and Tissue kit (QIAGEN Sciences Inc., Germantown, MD). Mosquito species identification was performed according to the protocols of Scott and others,15 Kengne and others,16,17 and Cohuet and others.18
For the mammalian blood meal identification, the assay by Kent and Norris19 was performed with minor modifications. Specifically, the concentration of the primers was reduced to 10 pmol for each 25-μL PCR, and the amount of DNA was increased to 1 μL. This multiplexed PCR allows for detection of human, cow, dog, pig, and goat blood meal sources based on the amplification of a cytochrome-b target sequence, which varies by size (Figure 1). MtDNA offers an ideal gene target for host species identification because of the high number of copies within cells and its high evolution rate,20,21 which allows for adequate interspecific variation to detect species differences while retaining sufficiently conserved flanking regions to facilitate universal primer design.22
Figure 1.
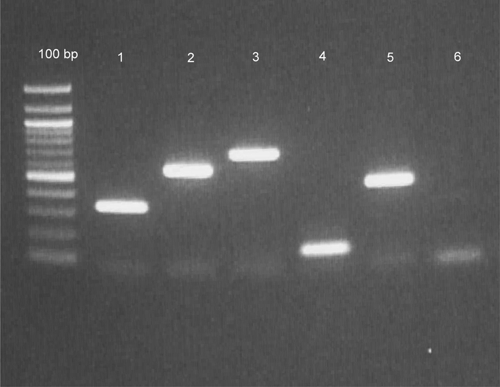
PCR assay for blood meal identification using the mtDNA cytochrome-b region. Lane 1 = human; lane 2 = cow; lane 3 = dog; lane 4 = goat; lane 5 = pig; lane 6 = negative control.17
The crude HBI was calculated using all the mosquitoes collected in the denominator. For the adjusted HBI, vectors with unidentified blood meal sources were excluded from the calculation. Human blood indices were calculated for each species. Mixed blood meals were included in the HBI calculation. For the statistical analysis, the SAS 9.1 software package was used, and the Fisher's exact test was performed. The level of significance was α = 0.05.
A total of 481 blood-fed mosquitoes were collected; of these, we could identify 471 individuals to species. The most abundant vector was An. gambiae s.s. (N = 275) followed by An. moucheti moucheti (N = 84). The remainder of analyzed mosquitoes included An. melas (N = 31) and members of the An. nili (N = 39) and An. funestus (N = 42) groups. The blood meal source was determined for 405 mosquitoes (Table 2). Failure to identify blood meal source could be caused by DNA degradation or preference for a host not detected by the assay.21 The majority of blood meals were derived from humans. Four mosquitoes took mixed blood meals (human and dog), and one took a blood meal derived from dog. The crude HBIs among the different mosquito species ranged from 0.56 to 1, whereas all adjusted HBIs were equal to 1 (Table 2). All of the mosquitoes collected from five locations on Bioko Island belonged to the An. gambiae s.l. complex and were analyzed as one collection site. These mosquitoes were used for the comparison between indoor and outdoor collections. On Bioko Island, a considerable proportion of blood-fed anopheline mosquitoes were collected in outdoor light traps (15.6%), and this was not much different from the proportion collected in indoor traps (21.4%). The blood meal contents from mosquitoes collected indoors (N = 21) versus outdoors (N = 24) did not differ significantly in their blood meal preference according to the Fisher's exact test (Fisher's exact test = 0.2; P = 0.469).
Table 2.
Blood meal source of collected vector species
| Vector species | Blood meal source | HBI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Human | Dog | Pig | Goat | Cow | Mixed source | N/A | Total | Crude HBI | Adjusted HBI | |
| An. gambiae s.s. | 228 (9) | 1 | – | – | – | 2 | 44 (1) | 275 (10) | 0.84 | 1* |
| An. melas | 22 (9) | – | – | – | – | 1 | 8 (5) | 31 (14) | 0.74 | 1 |
| An. m. moucheti | 81 | – | – | – | – | – | 3 | 84 | 0.96 | 1 |
| An. n. ovengiensis | 10 | – | – | – | – | – | 8 | 18 | 0.56 | 1 |
| An. carnevalei | 21 | – | – | – | – | – | – | 21 | 1 | 1 |
| An. leesoni | 33 | – | – | – | – | 1 | 8 | 42 | 0.81 | 1 |
| N/A | 5 | – | – | – | – | – | 5 | 10 | – | – |
| Total | 400 (18) | 1 | – | – | – | 4 | 76 (6) | 481 (24) | – | – |
N/A = no amplification of mtDNA target sequence. Numbers in parenthesis are outdoor-collected mosquitoes. All the numbers have been rounded to the second decimal point.
Actual arithmetic value is 0.999.
The HBI can be used to estimate the human biting habit, an integral component of vectorial capacity.1–3 Various studies have observed low HBI as a response to malaria control interventions.23,24 As such, HBI can be used to assess the effectiveness of malaria control programs. This index is simple and easy to measure, and it does not require the use of laborious and ethically challenging human landing captures to determine the human biting rate. When estimated periodically, the HBI, in conjunction with other malariometrics such as sporozoite and parity indices, can provide important information regarding the efficacy of antivector interventions.
Our results indicate that, on Bioko Island, mosquitoes collected indoors and outdoors have similar host feeding patterns. Ascertaining the representativeness of the sample is important, because biased sampling techniques may lead to overestimations of anthropophily.25 Taking this into account, the use of light traps was aimed to reduce such bias, because traps attract mosquitoes equally, regardless of host preferences. The relatively high proportions of blood-fed mosquitoes collected in light traps indicate that these traps may indeed be used successfully to collect engorged mosquitoes after they have fed and are seeking a resting place. As such, this method is useful for the collection of specimens for blood meal analysis studies, at least under conditions that prevail on Bioko Island. Additionally, past studies report that outdoor-fed An. arabiensis frequently rests indoors,26 suggesting that indoor-collected samples can also be representative of host feeding patterns.
In this study, An. gambiae s.s. was the most abundant vector, although this varied from site to site. Anopheles m. moucheti and An. carnevalei were more abundant in some hyperendemic sites. Despite being considered secondary vectors, the fact that these vector species remain highly anthropophilic in the face of ongoing intervention activities warrants further investigation. Traditional antivector methods such as IRS and LLINs specifically target endophilic and endophagic vectors and may not be optimal for non-An. gambiae vector suppression. As urbanization increases and human settlements expand in habitats that were previously considered sylvatic, the role of secondary vectors may become more important. Further study of host selection by these secondary vectors is warranted, given their proven abundance and anthropophily. Moreover, transmission venue studies should be performed before the selection of a particular malaria intervention method.
The high degree of anthropophily observed in the study is consistent with findings reported in other studies.27–31 Although some studies report zoophily in Afro-tropical vector populations,5–7 we did not detect such behaviors. This may be because of the scarcity of non-human hosts in collection sites that results from an observed lack of animal husbandry practices in Equatorial Guinea relative to other sub-Saharan African localities.32,33 In these situations, ongoing antimalarial interventions may not be enough to divert malaria vectors to non-human hosts. Different results may also be attributed to behavioral differences in local vector population or differences in collection methods. We recognize the limitations of our sampling scheme, which relied heavily on indoor collections. To reduce the inherent bias caused by indoor sampling, supplemental studies that specifically sample for outdoor resting mosquitoes should be performed in concert with indoor collections. Differences in host-seeking behavior may be caused by natural selection as opposed to excito-repellent effects caused by IRS or LLINs.
In this study, we observed a high degree of anthropophily in the vector population. This suggests that ongoing IRS activities may not be sufficient to reduce the HBI because of a variety of factors, including diminished residual insecticidal activity and household coverage and lack of available alternative vertebrate hosts. Additional studies should be conducted to examine whether the feeding patterns of An. m. moucheti and An. carnevalei should be taken into account in the design of malaria control interventions in locations where these vectors are abundant or may become abundant because of human activities. Finally, estimating the HBI can be a valuable tool for monitoring an important parameter useful in determining the efficacy of long-term antimalaria interventions.
ACKNOWLEDGMENTS
The authors thank Dr. Jeffrey Powell, Dr. Michel Slotman, Arcadio Edu, Dr. Abrahan Matias, Kirstin Dion, and Rafah Samir for their contributions in the field and laboratory. We also acknowledge Dr. Maria Diuk-Wasser, Dr. Anthony Kiszewski, and Dr. Johanna Daily for their editorial contributions. This study was supported by funds provided by the Medical Care Development International Inc. and Marathon Oil Corporation and by Grants AI046018 and AI046018 provided by the National Institutes of Health.
Footnotes
Authors' addresses: Vasiliki Pappa and Michael Reddy, Department of Epidemiology and Public Health, Yale University, New Haven, CT, E-mails: wassopappa@gmail.com and michael.reddy@yale.edu. Hans J. Overgaard, Medical Care Development International, Malabo, Equatorial Guinea and Norwegian University of Life Sciences, Aas, Norway. Simon Abaga, National Malaria Control Program, Ministry of Health, Republic of Equatorial Guinea, Malabo, Equatorial Guinea. Adalgisa Caccone, Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT.
References
- 1.Lardeux F, Loayza P, Bouchite B, Chavez T. Host choice and human blood index of Anopheles pseudopunctipennis in a village of the Andean valleys of Bolivia. Malar J. 2007;22:6–8. doi: 10.1186/1475-2875-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macdonald G. The Epidemiology and Control of Malaria. London, UK: Oxford University Press; 1957. [Google Scholar]
- 3.Garrett-Jones C. The Human Blood Index of malaria vectors in relation to epidemiological assessment. Bull World Health Organ. 1964;30:241–261. [PMC free article] [PubMed] [Google Scholar]
- 4.Muirhead-Thomson RC. Mosquito behaviour in relation to malaria transmission and control in the tropics. London: Edward Arnold & Co; 1951. pp. 56–70. [Google Scholar]
- 5.Sousa CA, Pinto J, Almeida AP, Ferreira C, do Rosario VE, Charlwood JD. Dogs as a favored host choice of Anopheles gambiae sensu stricto (Diptera: Culicidae) of São Tomé West Africa. J Med Entomol. 2001;38:122–125. doi: 10.1603/0022-2585-38.1.122. [DOI] [PubMed] [Google Scholar]
- 6.Teklehaimanot HD, Teklehaimanot A, Kiszewski A, Sacramento Rampao H, Sachs JD. Malaria in São Tomé and Principe: on the brink of elimination after 3 years of effective antimalarial measures. Am J Trop Med Hyg. 2009;80:133–140. [PubMed] [Google Scholar]
- 7.Duchemin JB, Leongpocktsy JM, Rabarison P, Roux J, Coluzzi M, Costantini C. Zoophily of Anopheles arabiensis and An. gambiae in Madagascar demonstrated by odour-baited entry traps. Med Vet Entomol. 2001;15:50–57. doi: 10.1046/j.1365-2915.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 8.Medical Care Development International EGMCI/ BIMCP. 2006. http://mcdi.mcd.org/current_malaria.htm Available at. Accessed March 30, 2010.
- 9.Kleinschmidt I, Torrez M, Schwabe C, Benavente L, Seocharan I, Jituboh D, Nseng G, Sharp B. Factors influencing the effectiveness of malaria control in Bioko Island, equatorial Guinea. Am J Trop Med Hyg. 2007;76:1027–1032. [PMC free article] [PubMed] [Google Scholar]
- 10.Sharp BL, Ridl FC, Govender D, Kuklinski J, Kleinschmidt I. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malar J. 2007;6:52. doi: 10.1186/1475-2875-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinschmidt I, Sharp B, Benavente LE, Schwabe C, Torrez M, Kuklinski J, Morris N, Raman J, Carter J. Reduction in infection with Plasmodium falciparum 1 year after the introduction of malaria control interventions on Bioko Island, Equatorial Guinea. Am J Trop Med Hyg. 2006;74:972–978. [PubMed] [Google Scholar]
- 12.Ridl FC, Bass C, Torrez M, Govender D, Ramdeen V, Yellot L, Edu AE, Schwabe C, Mohloai P, Maharaj R, Kleinschmidt I. A pre-intervention study of malaria vector abundance in Rio Muni, Equatorial Guinea: their role in malaria transmission and the incidence of insecticide resistance alleles. Malar J. 2008;7:194. doi: 10.1186/1475-2875-7-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohnstaedt LW, Gillen JI, Munstermann LE. Light-emitting diode technology improves insect trapping. J Am Mosq Control Assoc. 2008;24:331–334. doi: 10.2987/5619.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silver JB. Mosquito Ecology: Field Sampling Methods. New York, NY: Springer; 2008. pp. 372–492. [Google Scholar]
- 15.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 16.Kengne P, Antonio-Nkondjio C, Awono-Ambene HP, Simard F, Awolola TS, Fontenille D. Molecular differentiation of three closely related members of the mosquito species complex, Anopheles moucheti, by mitochondrial and ribosomal DNA polymorphism. Med Vet Entomol. 2007;21:177–182. doi: 10.1111/j.1365-2915.2007.00681.x. [DOI] [PubMed] [Google Scholar]
- 17.Kengne P, Awono-Ambene P, Antonio-Nkondjio C, Simard F, Fontenille D. Molecular identification of the Anopheles nili group of African malaria vectors. Med Vet Entomol. 2003;17:67–74. doi: 10.1046/j.1365-2915.2003.00411.x. [DOI] [PubMed] [Google Scholar]
- 18.Cohuet A, Simard F, Toto JC, Kengne P, Coetzee M, Fontenille D. Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. Am J Trop Med Hyg. 2003;69:200–205. [PubMed] [Google Scholar]
- 19.Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005;73:336–342. [PMC free article] [PubMed] [Google Scholar]
- 20.Tyler DD. The Mitochondrion in Health and Disease. New York, NY: VCH Publishers; 1992. pp. 95–146. [Google Scholar]
- 21.Mukabana WR, Takken W, Seda P, Killeen GF, Hawley WA, Knols BGJ. Analysis of arthropod blood meals using molecular genetic markers. Trends Parasitol. 2002;18:505–509. doi: 10.1016/s1471-4922(02)02364-4. [DOI] [PubMed] [Google Scholar]
- 22.Kocher TD, White TJ. In: PCR Technology: Principles and Applications for DNA Amplification. Erlich HA, editor. New York, NY: Stockton Press; 1989. pp. 137–147. [Google Scholar]
- 23.Loyola EG, Rodríguez MH, González L, Arredondo JI, Bown DN, Vaca MA. Effect of indoor residual spraying of DDT and bendiocarb on the feeding patterns of Anopheles pseudopunctipennis in Mexico. J Am Mosq Control Assoc. 1990;6:635–640. [PubMed] [Google Scholar]
- 24.Kaburi JC, Githuto JN, Muthami L, Ngure PK, Mueke JM, Mwandawiro CS. Effects of long-lasting insecticidal nets and zooprophylaxis on mosquito feeding behaviour and density in Mwea, central Kenya. J Vector Borne Dis. 2009;46:184–190. [PubMed] [Google Scholar]
- 25.Diatta M, Spiegel A, Lochouarn L, Fontenille D. Similar feeding preferences of Anopheles gambiae and An. arabiensis in Senegal. Trans R Soc Trop Med Hyg. 1998;92:270–272. doi: 10.1016/s0035-9203(98)91005-7. [DOI] [PubMed] [Google Scholar]
- 26.Tirados I, Costantini C, Gibson G, Torr SJ. Blood-feeding behaviour of the malaria mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol. 2006;20:425–437. doi: 10.1111/j.1365-2915.2006.652.x. [DOI] [PubMed] [Google Scholar]
- 27.Githeko AK, Service MW, Mbogo CM, Atieli FK, Juma FO. Origin of blood meals in indoor and outdoor resting malaria vectors in western Kenya. Acta Trop. 1994;58:307–316. doi: 10.1016/0001-706x(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 28.Okwa OO, Akinmolayan FI, Carter V, Hurd H. Transmission dynamics of malaria in four selected ecological zones of Nigeria in the rainy season. Ann Afr Med. 2009;8:1–9. doi: 10.4103/1596-3519.55756. [DOI] [PubMed] [Google Scholar]
- 29.Wanji S, Tanke T, Atanga SN, Ajonina C, Nicholas T, Fontenille D. Anopheles species of the mount Cameroon region: biting habits, feeding behaviour and entomological inoculation rates. Trop Med Int Health. 2003;8:643–649. doi: 10.1046/j.1365-3156.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- 30.Tanga MC, Ngundu WI, Judith N, Mbuh J, Tendongfor N, Simard F, Wanji S. Climate change and altitudinal structuring of malaria vectors in south-western Cameroon: their relation to malaria transmission. Trans R Soc Trop Med Hyg. 2010;104:453–460. doi: 10.1016/j.trstmh.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Magbity EB, Marbiah NT, Maude G, Curtis CF, Bradley DJ, Greenwood BM, Petersen E, Lines JD. Effects of community-wide use of lambdacyhalothrin-impregnated bednets on malaria vectors in rural Sierra Leone. Med Vet Entomol. 1997;11:79–86. doi: 10.1111/j.1365-2915.1997.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 32.International Livestock Research Institute International Livestock Center for Africa. 1992. http://agtr.ilri.cgiar.org/library/docs/x5474e/x5474e00.htm#Contents Available at. Accessed July 13, 2010.
- 33.Albrechtsen L, Fa JE, Barry B, Macdonald DW. Contrasts in availability and consumption of animal protein in Bioko Island, West Africa: the role of bushmeat. Environ Conserv. 2006;32:340–348. [Google Scholar]


