Abstract
A seroepidemiological investigation was conducted among the population of two municipalities in Northeastern Brazil. Immunoglobulin M (IgM) and IgG antibodies to Burkholderia pseudomallei were positive in 51.27% (161 in 317 samples) and 58.49% (186), respectively. IgM titers were higher in children than in adults. On the contrary, IgG increased progressively with age. We observed a significant association between agricultural occupation and raised IgM titers (P < 0.005) and IgG titers (P < 0.001), and between construction workers and raised IgG titers (P = 0.005). Antibody IgG avidities did not correlate with age. The highest titers of antibodies (1/800) showed the highest antibody avidity indexes (P < 0.01). Most of the serum samples recognized 45-kDa and 200-kDa bands by IgG1 and IgG2 subclasses. Our study showed a high seropositivity among individuals living in endemic regions of the state of Ceará, and highlights the need for further surveillance close to water courses such as dams and rivers in Northeastern Brazil.
Introduction
Melioidosis is a fulminating and potentially fatal infectious disease caused by the facultative intracellular Gram-negative bacterium, Burkholderia pseudomallei. The infection includes a cluster of clinical syndromes covering a broad spectrum from asymptomatic conditions to acute septicaemia or pneumonia. Burkholderia pseudomallei can be recovered from soil and fresh surface water and survives under hostile environmental conditions, including a prolonged lack of nutrients.1 Cases are reported predominantly in Southeast Asia and northern Australia. In Brazil, a cluster of cases was first reported in the Municipality of Tejuçuoca, Ceará state in 2003, when three of four children from the same family died of multiple organ systems failure caused by the infection.2 Antibody seropositivity can be demonstrated in the healthy population of endemic areas, indicating subclinical infection,3 and not necessarily a clinically evident disease state.4 The culture filtrate of B. pseudomallei is rich in secretory antigens mainly composed of exopolysaccharides, lipopolysaccharides (LPS), and proteins and is considered to be a source of antigen for a reliable and sensitive serological method for melioidosis diagnosis in endemic areas.5 In this study, we aimed to discover the extent of exposure to B. pseudomallei in the population of endemic areas of the state of Ceará, Brazil by a targeted seroepidemiological investigation.
Subjects and methods
A seroepidemiological study was conducted from February to August, 2006, in the municipalities of Tejuçuoca and Banabuiu,6 Ceará, where case clusters of melioidosis occurred previously. A questionnaire was administered to the participants of the study (N = 321), who resided in one of those localities. This included 104 participants living alongside the Banabuiu River in Banabuiu, and 217 participants living alongside the Caxitore River in Tejuçuoca. The epidemiological investigation sought information on demographic variables (age, gender, residence locality), previous disease history, contact with water (clothes washing, occupational or leisure activities) and soil (civil construction, agriculture, gardening). Other kinds of occupations (e.g., student, housewife) were also recorded. All the participants were clinically healthy and only one person had known melioidosis in the past.
The study was approved by the Ethics Committee of the Federal University of Ceará under process no. 16/2005. Informed consent was obtained from each participant before blood collection.
A 2 mL volume of blood was collected from each participant and, after centrifugation, the serum was sent to a reference laboratory where it was kept at −20°C until analysis.
Antigen preparation.
The B. pseudomallei strain used in this study was isolated from blood culture of a patient with septicaemic melioidosis and confirmed by phenotypic and molecular methods, according to validated discovery pathway.7 Burkholderia pseudomallei was inoculated into separate flasks of protein-free media and incubated at 37°C for 2 weeks. The culture was mixed twice each day. The culture broth was then autoclaved at 115 lbs pressure (121°C) for 15 min.8 The material was filtered through filter paper. Saturated ammonium sulphate was added, leaving 24 h for precipitation. The material was centrifuged at 10,000 rpm for 30 min and, after dialysis against saline solution; it was kept at −20°C.
Serum anti-B. pseudomallei IgG and IgM titers.
Briefly, microplates (Costar, Cambridge, MA) were coated with 50 ng/well of crude extract of B. pseudomallei. After 16 h at 4°C, the plates were incubated with four serially diluted serum samples (in duplicates) from 1:100 in phosphate buffered saline (PBS)-containing 0.5 M and 0.2% Tween 20. After 1 h 30 min at 37°C, the plates were washed four times with PBS containing 0.05% Tween 20 and incubated with 1:4000 dilution anti-human IgG or anti-human IgM-peroxidase conjugates (Sigma, St. Louis, MO). After 1 h at 37°C, the plates were washed and incubated with a substrate solution containing 0.4 mg/mL orthophenylenediamine in citrate-phosphate buffer, 0.1 M, pH 5.0, and 0.01% H2O2 final concentration. After 30 min, the color development was interrupted by the addition of 2.5 N H2SO4. Reading was done at 492 nm. The results were expressed in titers. The cut-off value was considered as the mean of the optical density readings of a negative control.
The enzyme-linked immunosorbent assay (ELISA) was also performed on 20 serum samples from Australian individuals with negative results by the indirect passive hemagglutination technique.
Serum anti-B. pseudomallei IgG and IgM avidities.
The avidity of antibodies was determined using potassium sodium thiocyanate (KSCN) to elute the bound complexes.9 Briefly, serum samples were incubated in the antigen-coated microplates, as described previously, after choosing the dilution that had presented an absorbance of at least 0.800. After washings, KSCN was added to the wells in various concentrations (0.0; 0.10; 0.25; 0.5; 1.0; 2.0; 3.0; 4.0 M) during 30 min at room temperature. The plates were then washed 6 times and incubated with 1:4000 diluted anti-human IgG-peroxidase conjugates (Sigma). After 1 h at 37°C, the plates were washed and incubated with the substrate solution, as described previously. The results were expressed by the molar concentration of KSCN necessary for elution of 50% of the bound immune complexes.
Western blot analysis.
The crude extract from B. pseudomallei was pre-treated with a digestion solution containing sodium dodecyl sulphate and beta-mercaptoethanol before its electrophoresis in 10.0% sodium dodecyl sulphate-polyacrylamide gels. The proteins were electrophoretically transferred onto nitrocellulose membranes. The membranes were blocked with 5% skim milk in Tris-buffered saline at room temperature for 2 h, and then incubated with serum samples diluted to 1:100 in 5% skim milk/Tris/0.1% Tween 20 for 16 h at room temperature. For the IgG subclass detection, the membranes were washed after serum incubation and incubated with anti-human IgG1 (clone HP 6012, Center for Disease Control and Prevention, Atlanta, GA), anti-human IgG 2 (clone HP 6002), anti-human IgG3 (clone HP 6050), anti-human IgG4 monoclonals (clone HP 6101) diluted to 1:1000 in 1% skim milk/Tris/0.1% Tween 20. After three washes, the membranes were incubated at room temperature for 1 h with alkaline phosphatase conjugated anti-mouse IgG diluted to 1% skim milk/Tris/0.1% Tween 20 (Sigma).
Statistical analysis.
Data were analyzed using Epi Info (CDC, Atlanta, GA) and STATA 7.0 (Stata Corp., College Station, TX). Differences in proportions were compared by the χ2 test. For comparison of antibody titers and avidities, the Spearman correlation test was used, and for comparison of the antibody avidity in the different age groups, the Kruskal-Wallis and the Dunn tests were used. These data were analyzed by the Graph Pad Instat 3.06 and by the Graph Pad Prism 4.00.255 programs (Graph Pad Software Inc., San Diego, CA).
Results
Serum anti-B. pseudomallei IgG and IgM titers.
The seroprevalence of total antibodies against B. pseudomallei found in the study was 80.8% (256 in 317 samples). The IgM and IgG antibody titers were positive in 51.3% (161 in 317 samples) and 58.5% (186), respectively. The distribution of age range showed higher IgM antibody titers in children than in adults. IgG, on the other hand, increased progressively with age (Figure 1). In respect to the type of occupational exposure, we observed a significant association between agriculture and raised IgM titers (44.2%, P < 0.005) and IgG titers (69.1%, P < 0.001). Another significant occupational association was between construction workers and IgG titers (84.6%, P = 0,005). No statistical difference was observed between genders, or among people living in the two municipalities and their 6 neighborhoods (4 in Tejuçuoca and 2 in Banabuiu). No association was found with contact with dam water or irrigation. In respect to the antibody IgG avidity, there was good correlation between antibody IgG avidity and the age range from 4 to 40 years (r = 0.21, P = 0.02, Figure 2), but not at a higher age range (r = 0.03, P > 0.5). Moreover, there were higher avidity indexes in titers 1/800 than 1/200 and 1/ 400 (Kruskall-Wallis test, P < 0.001, Figure 3).
Figure 1.
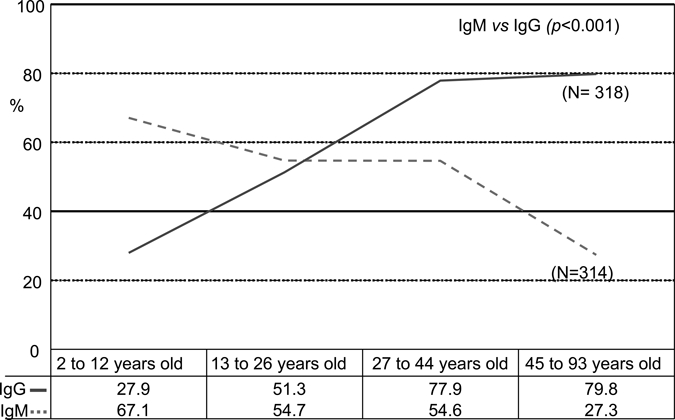
Percentage of positive (IgG) and IgM against a crude extract of Burkholderia pseudomallei IgG and IgM in serum samples from individuals residing in Tejuçuoca and Banabuiu, Ceará state, Brazil, according to the age range.
Figure 2.
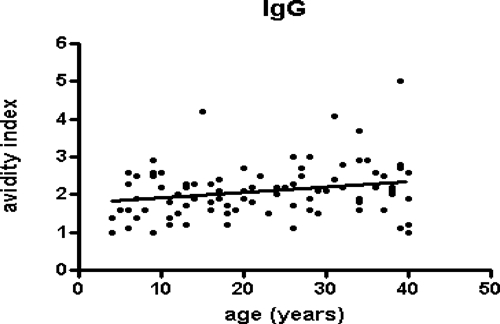
Antibody IgG avidity indexes against a crude extract of Burkholderia pseudomallei in serum samples from individuals residing in Tejuçuoca and Banabuiu, Ceará state, Brazil, according to the age range. The results were expressed by the molar concentrations of potassium sodium thiocyanate (KSCN) necessary for elution of 50% of the bound immune complexes. The traces represent the avidity index mean for each group.
Figure 3.
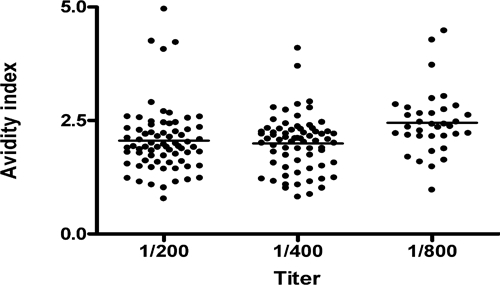
Antibody IgG avidity indexes against a crude extract of Burkholderia pseudomallei in serum samples from individuals residing in Tejuçuoca and Banabuiu, Ceará state, Brazil, according to the titers of the IgG antibodies. The results were expressed by the molar concentrations of potassium sodium thiocyanate (KSCN) necessary for elution of 50% of the bound immune complexes. The traces represent the avidity index mean for each group.
Two samples with positive IgG titers and one sample with positive IgM and IgG titers were found in the Australian group.
IgG subclass analysis by Western blotting.
The five high-titer samples tested showed detectable bands on IgG subclass analysis. Three samples presented antibodies that recognized antigenic bands at 45-kDa position and two showed reactivity to the 200-kDa band. The only subclasses present in the samples were IgG1 and/or IgG2 (Figure 4). No sample presented IgG3 or IgG4 (data not shown).
Figure 4.
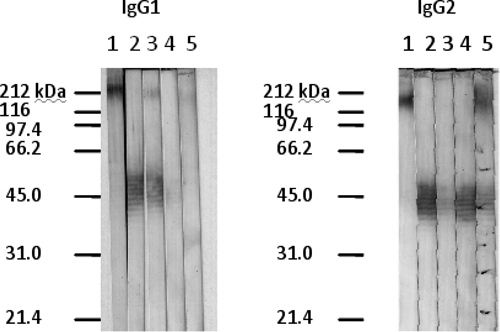
Detection of IgG1 and IgG2 subclasses against Burkholderia pseudomallei culture filtrate antigens by the Western blot technique employing serum samples from five individuals living in Banabuiu and Tejuçuoca. Lane 1 represents a high-tittered sample in enzyme-linked immunosorbent assay.
Discussion
The B. pseudomallei serology is an important epidemiological tool for use in association with isolation of the organism from the soil in endemic regions,10 although its usefulness in clinical situations is not as clear.1 The presence of antibodies, mainly represented by anti-LPS antibodies,3 is considered to be the result of environmental exposure to the bacterium.
Our group previously isolated the bacterium from the soil of Tejuçuoca and Banabuiu,11 two localities where melioidosis has been diagnosed. In this study, we found a seroprevalence of 51.3% for the IgM isotype, and 58.49% (186) for the IgG isotype. We cannot discard the possibility of false positive results in our ELISA assay once it was performed with a culture filtrate of B. pseudomallei, rather than purified antigens. Nonetheless, the finding of cross-reactivity does not diminish the importance of our study, which could show seropositivity among people living in endemic regions in Ceará. Additional studies with highly purified antigens are necessary to confirm our current data. Wuthiekanun and others10 found a seroprevalence of 16% among children between birth and 16 years of age in Cambodia, and positivity rose with age. Our observations that IgM levels decreased with age, whereas the IgG levels rose with age, supports the view that serology results at an early age is more representative of recent bacterial infection, and at later ages is more likely to represent a chronic infection state. Ashdown4 showed that only patients with clinical melioidosis had detectable IgM antibodies, which remained positive for a variable period, from 3 months to more than 2 years. On the other hand, Chenthamarakshan and others5 found elevated levels of IgM antibodies in their control group, ranging from 1:50 to 1:3,200 titers, whereas the risk group showed titers below 1:100. We considered a cut-off of 1:200 for both IgM and IgG to diminish false-positive results. According to Chenthamarakshan and others,12 elevated IgM levels can be found in healthy controls, probably caused by continuous exposure to the organism in the environment. The use of different methodologies in the studies does not allow direct comparisons with our data.
The strong correlation observed between agriculture, construction workers, and serum antibody positivity reinforces the observation that the contact with soil frequently exposes workers to the bacterium, but does not necessarily induce disease development, as seen in this work.
In regard to the B. pseudomallei culture filtrate antigen preparation, it has been demonstrated that the crude extract shows good correlation with antigen solutions containing lipopolysaccharide (LPS) and solutions containing both LPS and exopolysaccharide, but not with exopolysaccharide alone, suggesting that the immunodominant epitope was LPS.13 The crude antigen for ELISA has been considered a good tool for seroepidemiological purposes because the antigen is simple to obtain. The immunoblotting analysis of our serum samples revealed reactivity at the 200-kDa position, at the 45- to 68-kDa range, at the 38-kDa position, and lower than the 21-kDa position. The 200-kDa antigen is an exopolysaccharide,14 that is, a capsular polysaccharide, which contains a 3-deosy-D-manno-2-octusolonic acid residue (KDO), a component present in LPS and capsular polysaccharides of Gram-negative bacteria, such as Escherichia coli.15 The bands ranging from 45- to 68-kDa represent reactivity to the repeating polysaccharide O-side chain of the LPS and the band below the 21-kDa position reactivity to the lipid A core oligosaccharide of the LPS.16 The reaction to a band appearing at the 38-kDa position may result from nonspecific interactions with a component of the bacterium.14
We found that IgG1 and IgG2 were the predominant antibody subclasses reactive to the culture filtrate of B. pseudomallei. They are considered to be the predominant subclasses involved in the humoral immune response to human B. pseudomallei infection.12 The protective function of these antibodies is directly associated with their avidity properties, as occurs for other microorganisms.17,18
Finally, we conclude that in localities (Tejuçuoca and Banabuiu), where a cluster of meliodosis cases has been previously diagnosed, positivity of serum samples in individuals living in the endemic regions has been verified. This is mostly of the IgG1 and IgG2 subclasses against the O-chain of the LPS, and could be representative of subclinical infections. A high seropositivity among individuals living in endemic regions of the state of Ceará highlights the need for surveillance to melioidosis in Northeastern Brazil.
Footnotes
Financial support: This research was supported by Secretaria de Ciência, Tecnologia e Insumos Estratégicos (SCTIE) - Departamento de Ciência e Tecnologia (DECIT), Ministério da Saúde, Brasil; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ); Fundação Cearense de Amparo à Pesquisa (FUNCAP) and Secretaria da Saúde do Ceará (SESA).
Disclosure: The project was approved by the Ethical Committee of Walter Cantídio University Hospital, Universidade Federal do Ceará, on 31 January 2005.
Authors' addresses: Dionne Bezerra Rolim, Universidade de Fortaleza, Brazil, E-mail: dionnerolim@gmail.com. Dina Cortez F. L. Vilar, Epidemiology Group, Health Department of Ceará, Fortaleza, CE, Brazil, E-mail: dina@saude.ce.gov.br. Luciano Pamplona de Góes Cavalcanti, Department of Community Health, Federal University of Ceará, Brazil, E-mail: pamplona.luciano@gmail.com. Liara B. N. Freitas and Aparecida Tiemi Nagao-Dias, Department of Clinical Analysis and Toxicology, Federal University of Ceará, Brazil, E-mails: lyara.freitas@hotmail.com and tiemindi@yahoo.com.br. Timothy J. J. Inglis, Division of Microbiology and Infectious Diseases, PathWest Laboratory Medicine WA, QEII Medical Centre, Nedlands, Perth, Western Australia, Australia, E-mail: tim.inglis@health.wa.gov.au. Jorge Luiz Nobre Rodrigues, Department of Clinical Medicine, Federal University of Ceará, Brazil, E-mail: jrodrigues@fortalnet.com.br.
References
- 1.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolim DB, Vilar DC, Sousa AQ, Miralles IS, Oliveira DC, Harnett G, O'Reilly L, Howard K, Sampson I, Inglis TJ. Melioidosis, northeastern Brazil. Emerg Infect Dis. 2005;11:1458–1460. doi: 10.3201/eid1109.050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anuntagool N, Intachote P, Wuthiekanun V, White NJ, Sirisinha S. Lipopolysaccharide from nonvirulent Ara+ Burkholderia pseudomallei isolates is immunologically indistinguishable from lipopolysaccharide from virulent Ara-clinical isolates. Clin Diagn Lab Immunol. 1998;5:225–229. doi: 10.1128/cdli.5.2.225-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashdown L. Relationship and significance of specific immunoglobulin M antibody response in clinical and subclinical melioidosis. J Clin Microbiol. 1981;14:361–364. doi: 10.1128/jcm.14.4.361-364.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chentamarakshan V, Vadivelu J, Puthucheary SD. Detection of immunoglobulins M and G using culture filtrate antigen of Burkholderia pseudomallei. Diagn Microbiol Infect Dis. 2001;39:1–7. doi: 10.1016/s0732-8893(00)00212-1. [DOI] [PubMed] [Google Scholar]
- 6.Inglis TJ, Rolim DB, Sousa AQ. Melioidosis in the Americas. Am J Trop Med Hyg. 2006;75:947–954. [PubMed] [Google Scholar]
- 7.Inglis TJ, Merritt A, Chidlow G, Aravena-Roman M, Harnett G. Comparison of diagnostic laboratory methods for identification of Burkholderia pseudomallei. J Clin Microbiol. 2005;43:2201–2206. doi: 10.1128/JCM.43.5.2201-2206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander AD, Huxsoll DH, Warner AR, Shepler V, Dorsey A. Serological diagnosis of human melioidosis with indirect hemagglutination and complement fixation tests. Appl Microbiol. 1970;20:825–833. doi: 10.1128/am.20.5.825-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagao AT, Carneiro-Sampaio MM, Carlsson B, Hanson LA. Antibody titer and avidity in saliva and serum are not impaired in mildly to moderately undernourished children. J Trop Pediatr. 1995;41:153–157. doi: 10.1093/tropej/41.3.153. [DOI] [PubMed] [Google Scholar]
- 10.Wuthiekanun V, Peaktra N, Putchhat H, Sin L, Sen B, Kumar V, Langla S, Peacock SJ, Day NP. Burkholderia pseudomallei antibodies in children, Cambodia. Emerg Infect Dis. 2008;14:301–303. doi: 10.3201/eid1402.070811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolim DB, Rocha MF, Brilhante RS, Cordeiro RA, Leitão-Junior NP, Inglis TJ. Environmental isolates of Burkholderia pseudomallei in Ceará State, northeast Brazil. Appl Environ Microbiol. 2009;75:1215–1218. doi: 10.1128/AEM.01953-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chenthamarakshan V, Kumutha MV, Vadivelu J, Puthucheary SD. Distribution of immunoglobulin classes and IgG subclasses against a culture filtrate antigen of Burkholderia pseudomallei in melioidosis patients. J Med Microbiol. 2001;50:55–61. doi: 10.1099/0022-1317-50-1-55. [DOI] [PubMed] [Google Scholar]
- 13.Chantatrita N, Wuthiekanun V, Thanwisai A, Limmathurotsakul D, Cheng AC, Chierakul W. Accuracy of ELISA using crude and purified antigens for the serodiagnosis of melioidosis. Clin Vaccine Immunol. 2007;14:110–113. doi: 10.1128/CVI.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anuntagool N, Sirisinha S. Antigenic relatedness between Burkholderia pseudomallei and Burkholderia mallei. Microbiol Immunol. 2002;46:143–150. doi: 10.1111/j.1348-0421.2002.tb02679.x. [DOI] [PubMed] [Google Scholar]
- 15.Nimtz M, Wray V, Domke T, Brenneke B, Häussler S, Steinmetz I. Structure of an acidic exopolysaccharide of Burkholderia pseudomallei. Eur J Biochem. 1997;250:608–616. doi: 10.1111/j.1432-1033.1997.0608a.x. [DOI] [PubMed] [Google Scholar]
- 16.Anuntagool N, Wuthiekanun V, White NJ, Currie BJ, Sermswan RW, Wongratanacheewin S. Lipopolysaccharide heterogeneity among Burkholderia pseudomallei from different geographic and clinical origins. Am J Trop Med Hyg. 2006;74:348–352. [PubMed] [Google Scholar]
- 17.Pontes GN, Massironi SG, Arslanian C, Palmeira P, Carneiro-Sampaio MM, Nagao AT. Human IgG but not IgM antibodies can protect mice from the challenge with live O6 Escherichia coli. Scand J Immunol. 2005;62:353–360. doi: 10.1111/j.1365-3083.2005.01669.x. [DOI] [PubMed] [Google Scholar]
- 18.Usinger WR, Lucas AH. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun. 1999;67:2366–2370. doi: 10.1128/iai.67.5.2366-2370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


