Abstract
Schistosomiasis is caused by parasitic trematodes. Individuals can accumulate hundreds of intravascular worms, which secrete a myriad of antigenic molecules into the bloodstream. Some of these molecules suppress immunity to microbial Toll-like receptor (TLR) ligands, such as lipopolysaccharides, which may increase host susceptibility to coinfecting pathogens. We show that schistosomiasis is associated with extremely high levels of endotoxemia as well as high mobility group 1, an endogenous inflammatory TLR ligand, in the absence of other coinfected pathogens. Circulating B cells express surface TLR2 and TLR4, reflecting systemic exposure to microbial ligands. Bacterial translocation may occur with schistosomal egg movement from the vascular to the gut and other routes, such as the skin during infection. Our report suggests that immunosuppressive schistosome antigens may have evolved to curb inflammatory responses to the high antigenic burden of translocated bacteria products and endogenous TLR ligands that arise during parasite exposure and inflammation.
Human schistosomiasis is a chronic inflammatory disease caused by intravascular parasitic trematodes. By day 2 post-infection in animal models, worms have entered the vasculature, where they have been shown to remain for up to 10 years in the human host secreting a myriad of molecules into the bloodstream, which modify multiple host cellular pathways.1 Individuals hyperexposed to parasitic schistosomes can accumulate hundreds of worms in their blood and thus, carry a significant antigenic burden. In particular, schistosomes produce several Toll-like receptor (TLR) ligands, including those for TLR2, TLR4, and TLR3.2–4
Evidence suggests that many schistosome-derived molecules, particularly TLR4 ligands and several glycoproteins, are immunosuppressive and down-regulate Th1 responses while actively promoting Th2-like immunity.5–7 For example, Schistosoma mansoni soluble egg antigens (SEAs) inhibit the activation of murine dendritic cells in response to lipopolysaccharide (LPS), CpG, and poly-I:C.8 Sm16 secreted by S. mansoni during skin penetration exerts potent inhibitory effects on the cytokine response to LPS and the exogenous TLR3 ligand, poly(I:C).9 SEA also suppresses LPS and polyI:C-mediated maturation and production of interleukin (IL)-12, IL-6, and tumor necrosis factor (TNF)-α by human dendritic cells.10 These observations support the hypothesis that schistosomiasis increases susceptibility to coinfecting pathogens.11 However, immunosuppression by schistosome antigens may also be clinically relevant in the context of blood-borne non-pathogenic bacteria.
Chronic inflammatory diseases, including inflammatory and primarily metabolic or infectious diseases, are associated with increased translocation of bacteria into the bloodstream, which can lead to unremitting stimulation of the immune system.12 Chronic exposure to systemic bacteria can result from mucosal ulceration of the gastrointestinal tract in inflammatory bowel disease (IBD) or altered intestinal immune responsiveness, such as in progressive human immunodeficiency virus (HIV) disease.13,14 A disturbed microcirculation and edematous gut wall in heart failure also allows bacteria to enter the bloodstream.15 Any of these mechanisms could occur in schistosomiasis because of altered circulation from eggs lodging in the vasculature, disrupted gut epithelium and endothelium from egg translocation, or inherently suppressed immunity from schistosomiasis itself or coinfections, such as HIV.16 In addition, individuals at risk, and particularly hyperexposed populations, may also be exposed to bacterial or other microbial antigens within infested water or their skin microflora, both of which might be transported in with infecting cercaria.17 Microbial TLR ligands also have the propensity to release of a host of endogenous TLR stimulatory ligands.18 These conditions set the host up for the accumulation of a myriad of systemic schistosome, microbial, and host-derived antigens.
We previously showed that chronic disease is associated with activated TLR2- and TLR4-expressing B cells in the periphery.19–21 These activated B cells can secrete significant amounts of cytokines and chemokines while in circulation.19 However, systemic host- and microbial-derived TLR ligands can modify B-cell responses in a pro- or antiinflammatory manner.22 This has been shown primarily by the differential responses of human B cells to TLR4 ligands.20,22 Interestingly, in patients with chronic disease, persistent endotoxemia does not necessarily associate with systemic inflammation and poor health.22 This may be related to the type of LPS in the bloodstream. The number of acyl chains on lipid A, the inflammatory moiety of LPS, can dictate whether LPS is agonistic or antagonistic.23 For example, lipid A from Escherichia coli is hyperacylated with six acyl chains that mediate inflammatory pathways through TLR4. In contrast, lipid A from various species of commensal bacteria may be hypoacylated and act in an antiinflammatory manner.23 In fact, in Crohn's disease (CD), systemic LPS may be immunosuppressive for B cells through a reduced systemic LPS lipid A acylation burden.22
We sought to determine if B cells were modified in human schistosomiasis and the potential effect of this disease on measurable TLR ligands in the bloodstream. We assessed the systemic TLR ligand burden and TLR expression by circulating B cells in a cohort of multiply treated occupationally exposed car washers and fisherman in Western Kenya.16 This study was approved by the Scientific Steering Committee of the Kenya Medical Research Institute, the National Ethics Review Board of Kenya, and Boston University Institutional Review Board and was performed in western Kenya along the shores of Lake Victoria as previously described.4 Study participants (N = 47 total) were aged 21–63 years (mean age ± standard deviation = 35.6 ± 12.5 years) and considered hyperexposed to S. mansoni, because they stand in the water for several hours a day either washing cars or fishing. Eggs per gram of feces (EPG) ranged from 0 to 2,880 (mean EPG ± standard deviation = 308 ± 664 EPG). Uninfected Kenyan subjects were recruited from the Kenya Medical Research Institute (N = 5). Uninfected/unexposed subjects (N = 50) were recruited from Boston, MA, as previously described.19 On informed consent, all blood samples were drawn into endotoxin-free, non-reactive heparin-containing tubes (BD Vacutainer with sodium heparin #366480; Becton Dickson, San Jose, CA) (Figure 1A). Plasma was stored at −80°C until use. Whole blood was evaluated by flow cytometry for expression of TLR4 and TLR2 on circulating CD19 + B cells. Briefly, 100 μL of blood were incubated with fluorescently labeled antibodies (anti-CD19) purchased from BD Pharmingen (San Jose, CA) and eBioscience (San Diego, CA; anti-TLR2 and anti-TLR4). Red blood cells were lysed with FACS Lysis Buffer (BD Pharmingen). Assessment of surface expression on B cells was performed with gates generated with anti-CD19 plus the appropriate isotype controls for each sample.24 Endotoxin was measured in the plasma with limulus amebocyte lysate (LAL) assay (Lonza, Basel, Switzerland) as previously described for other chronic inflammatory diseases.22 Plasma high-mobility group box 1 (HMGB1) levels were measured by enzyme-linked immunosorbent assay (ELISA; Shino-Test Corporation, Kanagawa, Japan). Stool samples were examined for S. mansoni eggs and other helminth ova by the modified Kato–Katz method (Vestergaard Frandsen; two slides each from three stool specimens obtained over several days). Subjects positive for S. mansoni were treated with 40 mg/kg praziquantel, and those positive for other helminth ova were treated with 400 mg of albendazole as previously described.24 Although some study participants were schistosome egg negative at the time of blood draw (n = 5), they were actively exposed to infective cercaria in Lake Victoria. Sample sizes of readouts varied because of incomplete availability of certain subject data.
Figure 1.
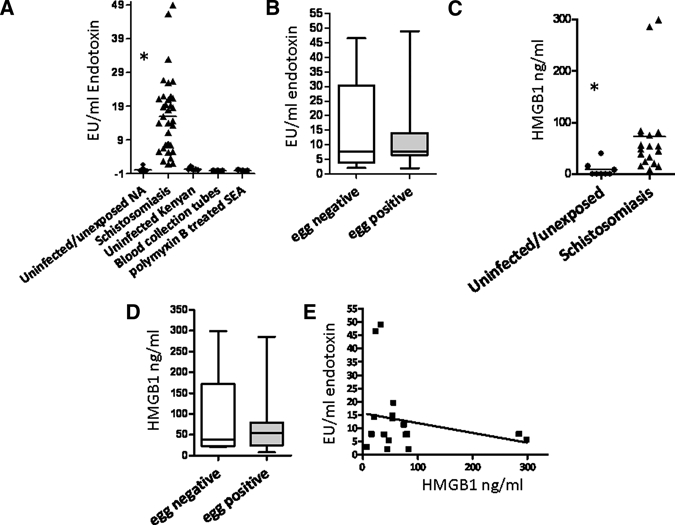
Schistosomiasis is associated with a high TLR ligand burden. (A) Plasma endotoxin levels are increased in occupationally hyperexposed individuals (N = 36) compared with uninfected/unexposed North Americans (N = 15; P < 0.0001) and uninfected Kenyans (N = 5). Blood collection tubes rinsed with phosphate-buffered saline (PBS) did not induce a false-positive reaction on the limulus amebocyte lysate (LAL) assay (N = 3). Polymyxin B-treated SEAs do not react with the LAL assay (N = 3; separate measures). (B) Levels of plasma endotoxin were not associated with fecal egg positivity in hyperexposed subjects: egg negative (N = 5); egg positive (N = 13); P = 0.805. Data are expressed as box plots, with the horizontal lines representing the 25th, 50th, and 75th percentiles. (C) Plasma high-mobility group box 1 (HMGB1) levels are elevated in hyperexposed subjects (N = 18) compared with uninfected/unexposed subjects (N = 8; P = 0.0006). (D) Plasma HMGB1 levels were not associated with fecal egg positivity in hyperexposed subjects: egg negative (N = 5); egg positive (N = 13); P = 0.621. Data are expressed as box plots, with the horizontal lines representing the 25th, 50th, and 75th percentiles. (E) There is no correlation between plasma endotoxin and HMGB1 levels in hyperexposed subjects (N = 18; r = −0.22; P = 0.365). Mann–Whitney U Test was used for differences in means and linear regression for correlation.
We found that hyperexposure to infectious schistosomes was associated with a high level of systemic endotoxin (Figure 1A). Significantly, levels of endotoxin were 10 times higher than the levels reported in lethal septic shock.25 This suggests that schistosome antigens can suppress the host response to the large dose of systemic LPS. Alternatively, the lipid A acylation burden may be reduced in schistosomiasis with an increased prevalence of hypoacylated LPS. Schistosome egg translocation from the mesenteric blood vessels to the gut lumen plays a role in systemic bacterial translocation in mice.26 We obtained EPG data on 18 of the study participants but found no difference in endotoxin levels between egg-positive and egg-negative subjects hyperexposed to schistosomes (Figure 1B). It is also possible that the LAL assay may detect blood-borne schistosome antigens. However, polymyxin B-treated egg antigens (gift from Edward J. Pearce, Trudeau Institute, Saranac Lake, NY) did not react with the assay (Figure 1A). Thus, a significant amount of endotoxin may enter the blood through other routes, such as the skin during cercarial penetration, and the anatomical site of bacterial origin might play a role in the inflammatory potential of LPS.17
LPS and other inflammatory mediators have been shown to induce the release of HMGB1.18 HMGB1 is an alarmin that mediates effects through its receptor for advanced glycation end product (RAGE) and has been implicated in the pathogenesis of septic shock.27,28 HMGB1 can also contribute to inflammation through TLR2, TLR4, and TLR9.29,30 Plasma HMGB1 levels were also quite high in the exposed cohort compared with unexposed/uninfected and were three times the level in IBD.22 Because SEAs can dampen responses of cells to LPS,31 we predicted that HMGB1 levels would be lower in egg-positive subjects. However, there was no difference between egg-positive and egg-negative study subjects, although fecal counts do not take into account liver egg or infecting cercariae burdens. The lack of a positive correlation between LPS and HMGB1 (Figure 1E) suggests that LPS in schistosomiasis does not promote the release of HMGB1 from cells; however, the data are cross-sectional. This may be suggestive of the LPS having characteristics of antagonistic lipid A or additional evidence that schistosomiasis has an immunomodulatory effect to the relatively large TLR ligand burden in the blood.
Because B cells can recirculate, TLR expression on peripheral blood B cells can reflect the inflammatory state of a patient as receptor levels may correlate directly with disease activity.19 Furthermore, schistosomes reside in the bloodstream and thus, can affect circulating cellular activation levels. We found that B cells expressed high levels of both TLR4 (Figure 2A and B) and TLR2 (Figure 2C). Thus, B-cell phenotypes are altered and resemble those from non-infectious chronic inflammatory diseases with high systemic levels of TLR ligands.19,22 However, in contrast to TLR2, B-cell TLR4 is not necessarily inflammatory and can function to suppress cytokine production through different TLR4 ligands than to which myeloid cells respond.22 Furthermore, this effect is disease-specific as B cells from patients with chronic inflammatory disease may respond to different TLR4 ligands.
Figure 2.
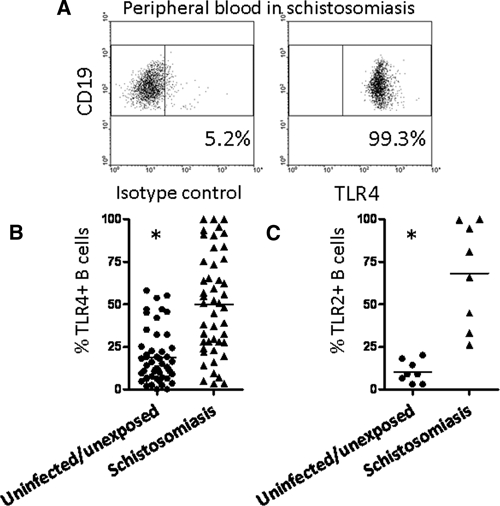
TLR4 and TLR2 expressions are elevated on circulating B cells in subjects hyperexposed to schistosomes. (A) TLR4 levels were assessed on CD19 + B cells in fresh whole blood. (B) The percentage of TLR4 + B cells is increased in hyperexposed subjects (N = 47) compared with the uninfected/unexposed North American cohort (N = 50; P < 0.0001). (C) The percentage of TLR2 + B cells is also increased in hyperexposed subjects (N = 8) compared with the uninfected/unexposed North American cohort (N = 8; P = 0.0002; Mann–Whitney U Test).
We show that schistosomiasis is associated with a high antigenic TLR ligand burden. The lack of relationship between LPS and HMGB1 suggests that, at least at the level of endotoxin and probably at the level of schistosome antigens, the TLR4 ligand burden in schistosomiasis has a null or antiinflammatory effect. Antiinflammatory LPS may come from specific species of bacteria or from host modification of lipid A, such as from neutrophil enzymes.32 Another intriguing possibility is that schistosomes themselves modify acyl chains of LPS, because they have been shown to alter acyl chains on phosphytidyl serine, to produce yet another array of TLR ligands.33 In contrast, HMGB1 can stimulate B cells to secrete IL-8 through TLR2 in CD and is likely inflammatory.22 Additional work needs to be done to determine its receptors and cellular distribution in schistosomiasis.
In conclusion, our report suggests that schistosomes may have evolved mechanisms to control host inflammation to other microbial antigens during infection. Understanding the full spectrum of the host immune response in schistosomiasis is critical to improving the health of people at risk for this disease. Our results may also open the door for the discovery of effective treatments for diseases that are associated with inflammatory endotoxin, such as septic shock or type 2 diabetes. In fact, cysteine proteases secreted from S. mansoni and another trematode parasite, Fasciola hepatica, protect mice from LPS-mediated septic shock by suppressing the release of nitric oxide, IL-6, TNF-α, and IL-12 from macrophages, showing the array of effects that schistosome antigens have on host immunity.34
ACKNOWLEDGMENTS
We would like to thank Elizabeth Achola, Kennedy Matunda, John Masa, John Oguso, and Boaz Mulonga for help with the laboratory work and all the study participants.
Footnotes
Financial support: This study was supported with funding from National Institute of Allergy and Infectious Diseases Grant A1074843 (to L.G.-L.) and Wellcome Trust Grant 08360 (to P.M.).
Authors' addresses: Daniel Onguru and Pauline Mwiniz, Vector Biology and Control Research Centre, Kenya Medical Research Institute, Kisumu, Kenya, E-mails: danonguru@yahoo.com and pmwinzi@kisian.mimcom.net. YanMei Liang, Qyana Griffith, and Lisa Ganley-Leal, Division of Infectious Diseases, Boston University School of Medicine, Boston, MA, E-mails: yanmei.liang@bmc.org, qyana@bu.edu, and Lisa.GanleyLeal@bmc.org. Barbara Nikolajczyk, Department of Microbiology, Boston University School of Medicine, Boston, MA, E-mail: bnikol@bu.edu.
References
- 1.Venugopal PG, Nutman TB, Semnani RT. Activation and regulation of toll-like receptors (TLRs) by helminth parasites. Immunol Res. 2009;43:252–263. doi: 10.1007/s12026-008-8079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Layland LE, Rad R, Wagner H, da Costa CU. Immunopathology in schistosomiasis is controlled by antigen-specific regulatory T cells primed in the presence of TLR2. Eur J Immunol. 2007;37:2174–2184. doi: 10.1002/eji.200737063. [DOI] [PubMed] [Google Scholar]
- 3.Aksoy E, Zouain CS, Vanhoutte F, Fontaine J, Pavelka N, Thieblemont N, Willems F, Ricciardi-Castagnoli P, Goldman M, Capron M, Ryffel B, Trottein F. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J Biol Chem. 2005;280:277–283. doi: 10.1074/jbc.M411223200. [DOI] [PubMed] [Google Scholar]
- 4.Thomas PG, Carter MR, Da'dara AA, DeSimone TM, Harn DA. A helminth glycan induces APC maturation via alternative NF-kappa B activation independent of I kappa B alpha degradation. J Immunol. 2005;175:2082–2090. doi: 10.4049/jimmunol.175.4.2082. [DOI] [PubMed] [Google Scholar]
- 5.Marshall FA, Pearce EJ. Uncoupling of induced protein processing from maturation in dendritic cells exposed to a highly antigenic preparation from a helminth parasite. J Immunol. 2008;181:7562–7570. doi: 10.4049/jimmunol.181.11.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Liempt E, van Vliet SJ, Engering A, Garcia Vallejo JJ, Bank CM, Sanchez-Hernandez M, van Kooyk Y, van Die I. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol Immunol. 2007;44:2605–2615. doi: 10.1016/j.molimm.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins SJ, Hewitson JP, Ferret-Bernard S, Mountford AP. Schistosome larvae stimulate macrophage cytokine production through TLR4-dependent and -independent pathways. Int Immunol. 2005;17:1409–1418. doi: 10.1093/intimm/dxh319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kane CM, Cervi L, Sun J, McKee AS, Masek KS, Shapira S, Hunter CA, Pearce EJ. Helminth antigens modulate TLR-initiated dendritic cell activation. J Immunol. 2004;173:7454–7461. doi: 10.4049/jimmunol.173.12.7454. [DOI] [PubMed] [Google Scholar]
- 9.Brannstrom K, Sellin ME, Holmfeldt P, Brattsand M, Gullberg M. The Schistosoma mansoni protein Sm16/SmSLP/SmSPO-1 assembles into a nine-subunit oligomer with potential to inhibit Toll-like receptor signaling. Infect Immun. 2009;77:1144–1154. doi: 10.1128/IAI.01126-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Riet E, Everts B, Retra K, Phylipsen M, van Hellemond JJ, Tielens AG, van der Kleij D, Hartgers FC, Yazdanbakhsh M. Combined TLR2 and TLR4 ligation in the context of bacterial or helminth extracts in human monocyte derived dendritic cells: molecular correlates for Th1/Th2 polarization. BMC Immunol. 2009;10:9. doi: 10.1186/1471-2172-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everts B, Adegnika AA, Kruize YC, Smits HH, Kremsner PG, Yazdanbakhsh M. Functional impairment of human myeloid dendritic cells during Schistosoma haematobium infection. PLoS Negl Trop Dis. 2010;4:e667. doi: 10.1371/journal.pntd.0000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki K. A study of endotoxemia in ulcerative colitis and Crohn's disease. II. Experimental study. Acta Med Okayama. 1978;32:207–216. [PubMed] [Google Scholar]
- 13.Pastor Rojo O, Lopez San Roman A, Albeniz Arbizu E, de la Hera Martinez A, Ripoll Sevillano E, Albillos Martinez A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:269–277. doi: 10.1002/ibd.20019. [DOI] [PubMed] [Google Scholar]
- 14.Douek D. HIV disease progression: immune activation, microbes, and a leaky gut. Top HIV Med. 2007;15:114–117. [PubMed] [Google Scholar]
- 15.Sandek A, Rauchhaus M, Anker SD, von Haehling S. The emerging role of the gut in chronic heart failure. Curr Opin Clin Nutr Metab Care. 2008;11:632–639. doi: 10.1097/MCO.0b013e32830a4c6e. [DOI] [PubMed] [Google Scholar]
- 16.Karanja DM, Hightower AW, Colley DG, Mwinzi PN, Galil K, Andove J, Secor WE. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet. 2002;360:592–596. doi: 10.1016/S0140-6736(02)09781-7. [DOI] [PubMed] [Google Scholar]
- 17.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JH, Kim SJ, Lee IS, Lee MS, Uematsu S, Akira S, Oh KI. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J Immunol. 2009;182:2458–2466. doi: 10.4049/jimmunol.0801364. [DOI] [PubMed] [Google Scholar]
- 19.Noronha AM, Liang Y, Hetzel JT, Hasturk H, Kantarci A, Stucchi A, Zhang Y, Nikolajczyk BS, Farraye FA, Ganley-Leal LM. Hyperactivated B cells in human inflammatory bowel disease. J Leukoc Biol. 2009;86:1007–1016. doi: 10.1189/jlb.0309203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagannathan M, Hasturk H, Liang Y, Shin H, Hetzel JT, Kantarci A, Rubin D, McDonnell ME, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. TLR cross-talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J Immunol. 2009;183:7461–7470. doi: 10.4049/jimmunol.0901517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin H, Zhang Y, Jagannathan M, Hasturk H, Kantarci A, Liu H, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. B cells from periodontal disease patients express surface Toll-like receptor 4. J Leukoc Biol. 2009;85:648–655. doi: 10.1189/jlb.0708428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonnell M, Liang Y, Noronha A, Coukos J, Kasper DL, Farraye FA, Ganley-Leal LM. Systemic toll-like receptor ligands modify B-cell responses in human inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:298–307. doi: 10.1002/ibd.21424. [DOI] [PubMed] [Google Scholar]
- 23.Coats SR, Reife RA, Bainbridge BW, Pham TT, Darveau RP. Porphyromonas gingivalis lipopolysaccharide antagonizes Escherichia coli lipopolysaccharide at toll-like receptor 4 in human endothelial cells. Infect Immun. 2003;71:6799–6807. doi: 10.1128/IAI.71.12.6799-6807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mwinzi PN, Ganley-Leal L, Black CL, Secor WE, Karanja DM, Colley DG. Circulating CD23+ B cell subset correlates with the development of resistance to Schistosoma mansoni reinfection in occupationally exposed adults who have undergone multiple treatments. J Infect Dis. 2009;199:272–279. doi: 10.1086/595792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Pedras-Vasconcelos JA, Brunet LR, Pearce EJ. Profound effect of the absence of IL-4 on T cell responses during infection with Schistosoma mansoni. J Leukoc Biol. 2001;70:737–744. [PubMed] [Google Scholar]
- 27.Pisetsky DS, Erlandsson-Harris H, Andersson U. High-mobility group box protein 1 (HMGB1): an alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res Ther. 2008;10:209. doi: 10.1186/ar2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devaraj S, Dasu MR, Park SH, Jialal I. Increased levels of ligands of Toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia. 2009;52:1665–1668. doi: 10.1007/s00125-009-1394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 30.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 31.Kane CM, Jung E, Pearce EJ. Schistosoma mansoni egg antigen-mediated modulation of Toll-like receptor (TLR)-induced activation occurs independently of TLR2, TLR4, and MyD88. Infect Immun. 2008;76:5754–5759. doi: 10.1128/IAI.00497-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gioannini TL, Teghanemt A, Zhang D, Prohinar P, Levis EN, Munford RS, Weiss JP. Endotoxin-binding proteins modulate the susceptibility of bacterial endotoxin to deacylation by acyloxyacyl hydrolase. J Biol Chem. 2007;282:7877–7884. doi: 10.1074/jbc.M605031200. [DOI] [PubMed] [Google Scholar]
- 33.van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt-Jones EA, Espevik T, de Jong EC, Kapsenberg ML, Golenbock DT, Tielens AG, Yazdanbakhsh M. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 34.Donnelly S, O'Neill SM, Stack CM, Robinson MW, Turnbull L, Whitchurch C, Dalton JP. Helminth cysteine proteases inhibit TRIF-dependent activation of macrophages via degradation of TLR3. J Biol Chem. 2010;285:3383–3392. doi: 10.1074/jbc.M109.060368. [DOI] [PMC free article] [PubMed] [Google Scholar]


