Abstract
Chagas cardiomyopathy remodeling is based on the presence of Trypanosoma cruzi in heart tissue and on the complex inflammatory response leading to a myocardium fibrosis and alterations in conductive and functional heart parameters. This study aims to evaluate Simvastatin on the inflammatory response and heart functionality using dogs infected with Y strain of T. cruzi. Animals were treated daily with Simvastatin (20 mg) for 6 months and submitted to clinical and immunopathological evaluations. Simvastatin reduced heart expression and serum levels of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) but not interleukin-10 (IL-10), possibly favoring blood parasitism but reducing inflammation and fibrosis in the left ventricle and right atrium. Simvastatin also ameliorated ejection fraction, diastolic diameter, and mass index of the left ventricle 6 months after infection. This study suggests that more investigation should be performed on the use of statins as a prophylactic therapy against cardiac remodeling because of their effects on modifying immune response and benefiting functional parameters in dogs with T. cruzi-induced ventricular dysfunctions.
Introduction
Chagas cardiomyopathy (CC) is a progressive inflammatory disease caused by the hemoflagellate parasite Trypanosoma cruzi that has caused around 2 million infected individuals in the Americas1 and been considered the most common form of non-ischemic cardiomyopathy worldwide.2 CC is initially marked by the presence of an inflammatory infiltration leading, in some years, to conductive and functional heart alterations.3 It is known that parasites per se or antigenic proteins are essential conditions to trigger and maintain the inflammatory response during CC.4,5 Pro-inflammatory cytokines interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) play a major role controlling tissue parasitism during T. cruzi infection, whereas regulatory cytokine interleukin-10 (IL-10) seems to moderate this response, suggesting a protective state to the infected host.6–8 Unfortunately, there is still no available pharmacologic therapy capable of eliminating parasites in the chronic phase of Chagas disease.9 In addition, because of its progressive chronic characteristic, the prognosis of CC is still poor and uncertain. Common and new therapies proposed in clinical treatment of CC aim to (1) maintain or recover the cardiac functions (e.g., β-blockers, angiotensin converting enzyme inhibitors, or digitalis) or (2) act on the genesis of inflammatory response and cardiac remodeling, because this remodeling may continue worsening clinical symptoms in CC.3
Experimental and clinical studies have indicated that 3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, commonly referred to as statins, present cardiovascular protective properties that compliment their lipid-lowering effects.10,11 Statins have also been shown to possess mammalian pleiotropic properties, including the ability to reduce systemic inflammation, stimulate angiogenesis, improve endothelial function, and mobilize bone marrow-derived stem cells.12–14 Evidence from several large clinical trials has shown that statins reduce morbidity and mortality in patients with distinct cardiovascular diseases, contributing to the improvement of left ventricular function and the prevention or attenuation of progressive left ventricular remodeling in heart failure.15,16
Taking advantage of the great clinical and pathologic similarity existing between human and dogs infected with T. cruzi,4,17 this study was designed to determine whether Simvastatin reduces inflammatory response in T. cruzi-infected mongrel dogs and protects the heart against CC remodeling during acute and recent chronic phases of Chagas disease.
Material and Methods
Parasites.
Blood trypomastigote forms of T. cruzi Y strain (described as T. cruzi II) were maintained through successive passages in Swiss mice at the Laboratory of Chagas disease, Universidade Federal de Ouro Preto (UFOP), Minas Gerais State, Brazil and later, were used in this study. We have previously shown that this strain presents distinct patterns of virulence and pathogenicity in the canine model of heart disease.18
Experimental animals and infection.
Fifteen (male and female) 4-month-old mongrel dogs from the Animal Facility at the UFOP were used in this study as mammalian hosts. The investigation conforms to the guidelines issued by the Brazilian College of Animal Experimentation (COBEA), and also, it was approved by the Ethic Committee in Animal Research (CEUA) at UFOP (number 2009/28). Animals were fed with commercial dog food and water ad libitum. Before this study, animals were dewormed and vaccinated against several infectious diseases. Ten dogs were inoculated with 2.0 × 103 bloodstream trypomastigotes (Y strain of T. cruzi) per 1 kg of body weight. The parasitemia of these animals was examined from the tenth day of infection and until the parasites were no longer detectable in fresh blood collected from the marginal ear vein. In parallel, five non-infected dogs were used as negative controls.
Reagent and scheme of treatment.
Monotherapy of Simvastatin (Sanval, São Paulo, SP, Brazil) at 20 mg/animal was given daily around 7 pm because of the circadian rhythms of this drug. A low dose of this pharmacology group was chosen because of its equivalence in dog and human therapies in preventing remodeling and progression of left ventricle dysfunctions.19–21 Animals were divided in three distinct groups: (1) five T. cruzi-infected dogs, (2) five T. cruzi-infected dogs treated daily with Simvastatin (20 mg), and (3) five non-infected dogs as a control group. One tablet of Simvastatin was placed on the oropharyngeal cavity, and the dog was observed for 10 minutes to avoid it spitting the drug. This therapy was initiated at the first day of T. cruzi inoculation, and it was continued for 6 months of infection, ending at the time of euthanasia of these animals.
Measurement of pro- and regulatory cytokines in serum.
For the detection of TNF-α, IFN-γ, and IL-10 (R&D systems, Minneapolis, MN) in plasma, 10-mL blood samples were collected monthly from each dog, and serum was stored at −80°C. Samples were defrosted and immediately processed using kits from R&D systems for dog cytokines. Briefly, flat-bottomed 96-well microtiter plates (Nunc) were coated with 100 μL/well of the appropriate monoclonal antibodies for 18 hours at 4°C and then washed with phosphate-buffered saline (PBS) buffer (pH 7.4) containing 0.05% Tween 20 (wash buffer). Non-specific binding sites were blocked with 200 μL/well of 1% bovine serum albumin (BSA) in PBS (blocking buffer). Plates were rinsed with wash buffer, and samples were added (100 μL/well) followed by incubation for 18 hours at 4°C. Plates were then washed, and 100 μL/well of the appropriate biotinylated detection antibodies diluted in blocking buffer containing 0.05% Tween 20 were added for 1 hour at room temperature. Plates were washed, streptavidin-horseradish peroxidase was added, and plates were incubated for 30 minutes at room temperature. Finally, plates were washed, and 100 μL/well of the chromogen substrate o-phenylendiamine (OPD; Sigma), diluted in 0.03 M citrate buffer containing 0.02% 30v/v H2O2, were added; after 30 minutes of a dark incubation at room temperature, the reaction was stopped by 50 μL/well of 1 M H2SO4 solution. Plates were read at 492 nm in a spectrophotometer (Emax; Molecular Devices). All samples were assayed in duplicate.
Semiquantitative analysis of IFN-γ and IL-10 expression in heart tissue.
RNA was isolated from the right atrium of dogs by acidic guanidinium thiocyanate–phenol–chloroform extraction. Polymerase chain reaction (PCR) conditions were as follows: 94°C for 5 minutes, 94°C for 1 minute, 57°C for 1 minute, 72°C for 2 minutes (three steps, n cycles), 57°C for 1 minute, and 72°C for 6 minutes (final extension). Primers (sense and antisense) sequenced from 5′ to 3′ are followed by the number of cycles and the expected product size of PCR according our previous standardization17 (shown in parentheses). IFN-γ: CGGCCTAACTCTCTGAAACG, CCTCCCTCTTACTGGTGCTG (38, 380 bp). IL-10: AGCACCCTACTTGAGGACGA, GATGTCTGGGTCGTGGTTCT (40, 249 bp). Hypoxanthine phosphoribosyltransferase (HPRT): AAGCTTGCTGGTGAAAAGGA, CAATGGGACTCCAGATGCTT (28, 219 bp; all primers from Dialab, Brazil). PCR products and molecular weight markers were run on 6% polyacrylamide gel and stained with silver nitrate. PCR products on silver-stained gels were quantified with a densitometer using a Quantity One program (The Discovery Series 1998; Biorad Laboratories). The densitometry values for each cytokine were divided by the average value for the HPRT for the same sample.
Doppler echocardiography.
Left ventricular parameters of heart dysfunction were evaluated at day 0 (before T. cruzi infection) and 3 and 6 months after infection, equivalent to acute and recent chronic phases of Chagas disease. Dogs were anesthetized by injection of sodic pentobarbital (Thiopentax, Cristália, SP, Brazil) at 0.5 mL/kg of body weight (0.03 g/mL in 0.89% saline solution), and all studies were performed on an Acuson Cypress Portable Ultrasound Machine (Siemens) and analyzed by an echocardiologist blinded to all clinical forms of the animals. More than 300 different parameters were collected and analyzed, but in this study, only left ventricle ejection fraction, left ventricle diastolic diameter, and mass index were shown. Animals were followed by researchers during the anesthesia recuperation, with no death registered during or after echocardiography evaluation.
Morphometric and histopathology analysis.
Animals were euthanized 6 months after the infection by an overdose of sodic pentobarbital (Thiopentax, Cristália, S, Brazil). Fragments of approximately 1.0 × 1.0 × 0.2 cm from the middle of the right atrial and left ventricle walls of each dog were taken for morphometric and histopathology analyses. Tissue fragments were fixed in 10% buffered formalin solution, dehydrated, cleared, and embedded in paraffin. Blocks were cut into 4-mm-thick sections and stained by hematoxylin and eosin (H&E) for inflammation assessment or Masson's trichromic for fibrosis quantitative evaluation. Twenty fields from each H&E- or Masson's trichromic-stained section were randomly chosen at a 40× magnification, giving a 1.5 × 106 μm2 analyzed myocardium area. Images were obtained through a Leica DM 5000 B microchamber (Leica Application Suite, version 2.4.0 R1) and processed by software Leica Qwin (V3) image analyzer. The inflammatory process was evaluated by a correlation index of the number of cell nuclei observed in the myocardium muscle from non-infected and infected animals (treated or untreated). This index corresponds to the nuclei of inflammatory leukocytes plus the background of cardiac cells nuclei observed in the non-infected dogs data.17 In this same way, fibrosis area was quantified using the image segmentation function. All pixels with blue hues in Masson's trichromic section were selected to build a binary image, subsequently calculating the total area occupied by connective tissue in non-infected and T. cruzi-infected and/or Simvastatin-treated dogs.
Statistical analysis.
The results of serological assays, cytokines expression, histological data, and echocardiography parameters were analyzed by non-parametric Newman-Keuls Multiple Comparison and Tukey tests. Difference was considered significant if P was equal to or less than 0.05.
Results
Simvastatin alters pattern of circulating parasites.
Low doses of statins did not alter circulating lipid levels in infected dogs treated with Simvastatin (N = 5) during 0, 3, and 6 months of T. cruzi infection, respectively, in mean ± standard error of the mean (SEM): (1) infected dogs (184.2 ± 19.79, 208.5 ± 31.93, and 190.9 ± 8.02 mg/dL) and (2) infected dogs plus Simvastatin (165.0 ± 10.71, 205.2 ± 8.98, and 180.3 ± 2.27 mg/dL). However, in terms of infection, a significantly higher parasitemia was observed between the days 11 and 14 after infection in animals treated with Simvastatin (Figure 1).
Figure 1.
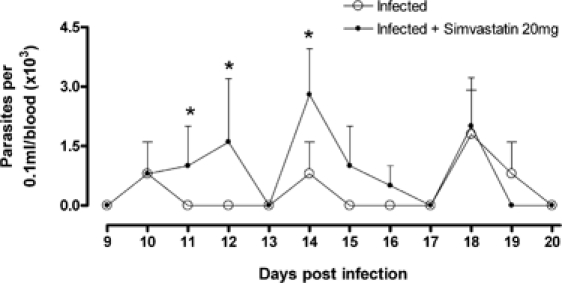
Parasitemia curve in dogs infected with T. cruzi treated with Simvastatin. Mongrel dogs were infected with the Y strain of T. cruzi and received daily low doses of Simvastatin (20 mg) or no drugs for 3–6 months; a representative parasitemia curve was shown before negativation on day 20. *P < 0.05 when both groups were compared at one specific point of the curve.
Low doses of Simvastatin alter pro-inflammatory cytokines in acute and chronic phases of Chagas disease.
Initial T. cruzi replication is partially controlled by pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-12, and oxygen derivatives (e.g., nitric oxide). Low doses of Simvastatin (20 mg/day) were capable of reducing serum levels of both pro-inflammatory TNF-α (Figure 2A) and IFN-γ (Figure 2B) around the first 2 months of infection but did not interfere in the regulatory cytokine IL-10 (Figure 2C). However, long-term therapy with this statin had an inversion of this systemic pattern of cytokines during the recent chronic phase of infection, showing a significant increase of IL-10 circulating levels but not the pro-inflammatory cytokines in the infected dogs treated with Simvastatin.
Figure 2.
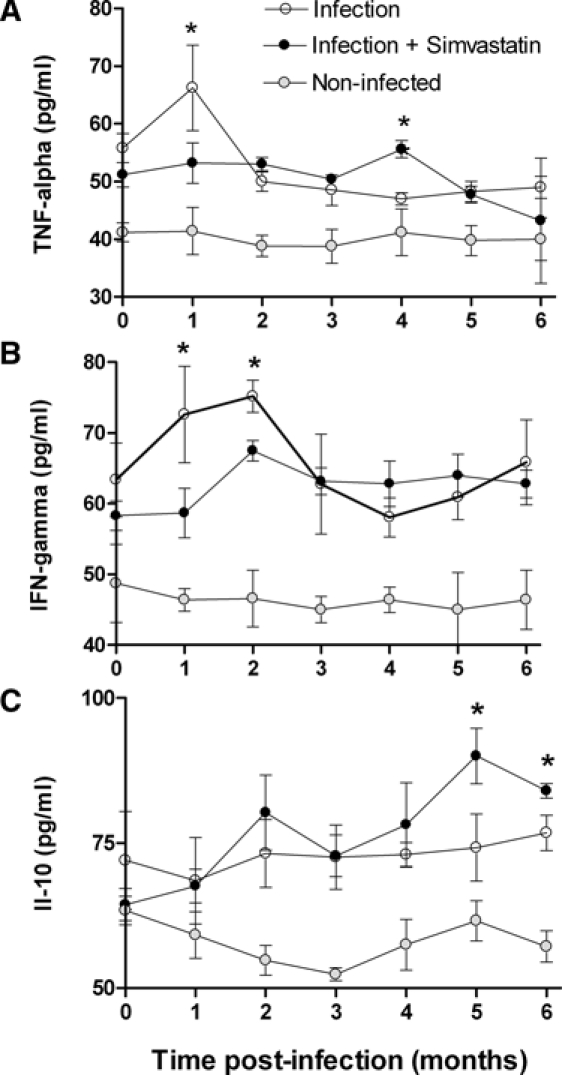
Concentrations of TNF-α, IFN-γ, and IL-10 in serum of infected dogs treated or not with Simvastatin. The concentrations of TNF-α (A), IFN-γ (B), and IL-10 (C) in serum of T. cruzi-infected mongrel dogs (N = 5) were measured by enzyme-linked immunosorbent assay (ELISA) monthly, and the mean of each point is given. *P < 0.05 when infected groups, treated or not with Simvastatin, were compared at one specific point of the curve.
During this recent chronic phase, animals were euthanized, and the expression of two representative Th1 (IFN-γ) and Th2 (IL-10) cytokines were measured in the heart tissue. Reinforced by our systemic data, messenger RNA (mRNA) expression of IL-10 (Figure 3B) was increased in those animals treated with Simvastatin, whereas the majority of IFN-γ (Figure 3A) was observed only in those infected and untreated dogs.
Figure 3.
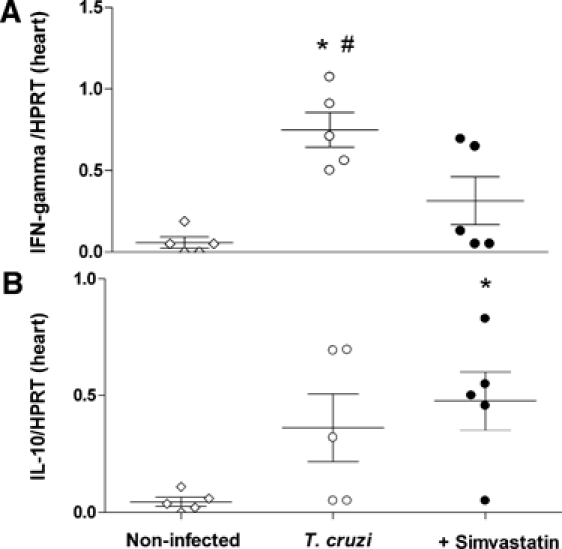
Pro-inflammatory and regulatory cytokine expression in heart tissue. The mRNA expressions of one pro-inflammatory cytokine, IFN-γ (A), and one regulatory cytokine, IL-10 (B), from T. cruzi-infected dogs untreated or treated with low doses of Simvastatin (20 mg) were analyzed. *P < 0.05 compared with non-infected animals. #P < 0.05 compared with animals treated with Simvastatin.
Histopathological findings after treatment with Simvastatin.
In this work, we did not observe evidence of amastigote nests in heart tissue of all evaluated groups. Comparisons among cellular nuclei from heart tissue derived from T. cruzi-infected dogs submitted to Simvastatin therapy or no therapy were made in the right atrium and left ventricle after 6 months of evaluation. Scattered infiltrating cells were observed in both treated and untreated infected dogs in the right atrium (Figure 4A and B) and left ventricle (Figure 5A and B), with no detectable abnormalities found in the vascular wall. A full analysis of this inflammatory pattern can be visualized in Figure 4E for the right atrium and in Figure 5E for the left ventricle, and regarding the volumetric proportions of the inflammatory process, the mean values were significantly higher in T. cruzi-induced chronic myositis than in those non-infected control animals.
Figure 4.
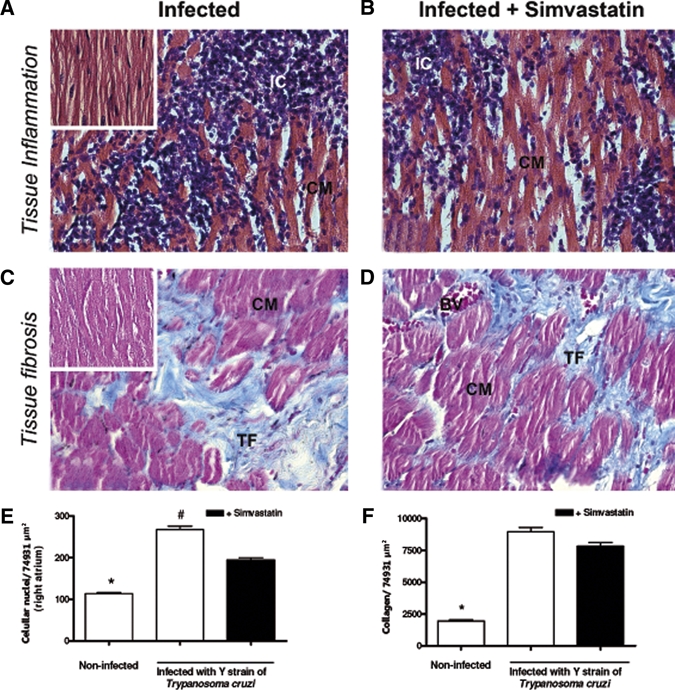
Simvastatin reduces inflammation in the T. cruzi-infected right atrium. Mongrel dogs infected with T. cruzi (A) and treated with Simvastatin (B) had their heart extracted on the month 6 after infection to evaluate inflammatory infiltration (H&E) in the right atrium segment. In parallel, it was observed that the intensity of collagen in those infected (C) and treated with Simvastatin (D) characterized fibrosis. Right atriums from non-infected dogs are illustrated by small images on the top of A and C, and the inflammatory infiltration (E) and collagen (F) in all groups were quantified in sections with 40× magnification. Results are given as the mean ± SEM. *P < 0.05 compared with infected animals. #P < 0.05 compared with infected animals treated with 20 mg of Simvastatin. IC = inflammatory cells; CM = cardiomyocites; TF = tissue fibrosis.
Figure 5.
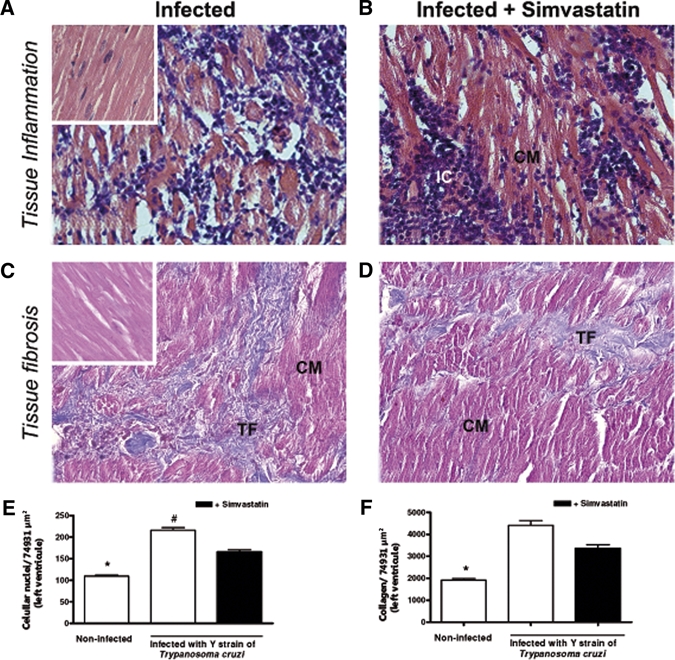
Simvastatin reduces the inflammation in T. cruzi-infected left ventricle. Mongrel dogs infected with T. cruzi (A) and treated with Simvastatin (B) had their heart extracted on month 6 after infection to evaluate inflammatory infiltration (H&E) in the right ventricle segment. In parallel, the intensity of collagen in those infected (C) and treated with Simvastatin (D) was observed to characterize fibrosis. The left ventricles from non-infected dogs are illustrated by small images on the top of A and C, and the inflammatory infiltration (E) and collagen (F), in all groups, were quantified in sections with 40× magnification. Results are given as the mean ± SEM. *P < 0.05 compared with infected animals. #P < 0.05 compared with infected animals treated with 20 mg of Simvastatin. IC = inflammatory cells; CM = cardiomyocites; TF = tissue fibrosis.
Interestingly, despite the capacity of Simvastatin to reduce the inflammatory infiltration (Figures 4E and 5E) in heart tissue, there were no differences in fibrosis in the right atrium (Figure 4C and D) and left ventricle (Figure 5C and D) between untreated infected dogs and those receiving 20 mg Simvastatin. The mean values of this quantification of collagen area in heart tissues are better observed in the right atrium in Figure 4F and in the left ventricle in Figure 5F.
Simvastatin preserves functional parameters in chronic Chagas cardiomyopathy.
Daily low doses of Simvastatin (20 mg) did not have significant effects on cardiac parameters at the end of the acute phase (3 months) of canine Chagas disease. However, in the recent chronic phase (6 months), it was observed to have a protective effect on the left ventricle ejection fraction (Figure 6A), diastolic end diameter (Figure 6B), and mass index (Figure 6C) in all infected dogs treated with Simvastatin. No significant differences were observed among non-infected animals during 3 and 6 months of evaluation for those evaluated parameters (data not shown).
Figure 6.
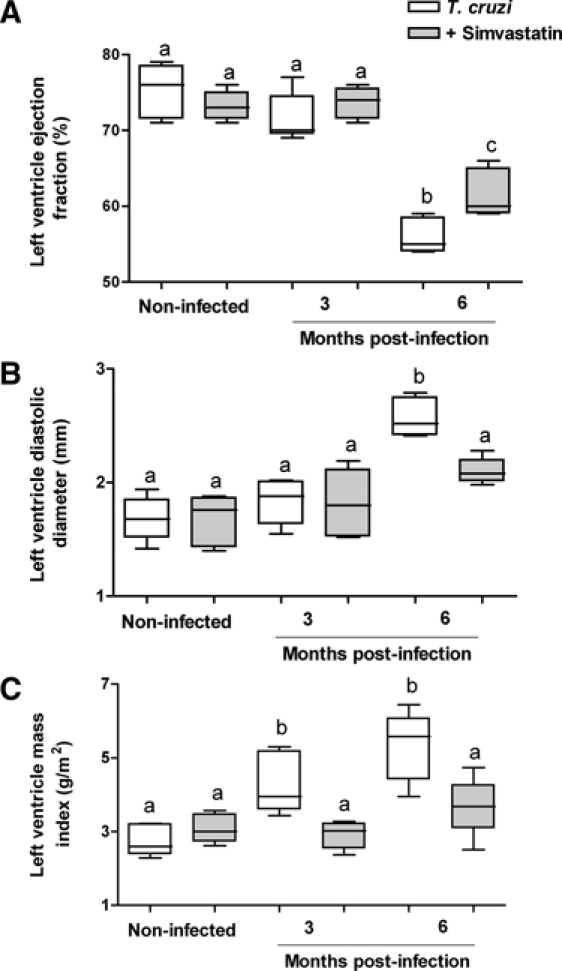
Simvastatin ameliorates heart parameters of the left ventricle in T. cruzi-infected animals. During three distinct moments of Chagas disease (before infection and 3 and 6 months after T. cruzi infection), mongrel dogs were submitted to echocardiogram analysis, and parameters of the left ventricular, such as ejection fraction (A), diastolic diameter (B), or mass index (C), were evaluated. Results are given as the mean ± SEM of individual groups: untreated dogs infected with T. cruzi (white box) and infected dogs treated with Simvastatin (gray box). The non-infected group maintained an unaltered pattern of ventricular function for 3 and 6 months. Letters indicate significant difference, and identical letters indicate similar values among infected groups untreated and treated with Simvastatin.
Discussion
Cardiac remodeling is best described as a process defined by structural changes in one or more cardiac chambers, particularly the ventricles. During CC, the cardiac remodeling is a pivotal process to define the progression to the mild or severe clinical forms of the disease. Underlying mechanisms are many fold; myocardial stretch and neurohormonal and cytokine activation are crucial processes in response to T. cruzi infection.4,22 Because heart injuries in CC seem to be partially defined by a common set of inflammatory responses, therapies aimed at counteracting these mechanisms might prove successful in attenuating or even preventing cardiac remodeling in the human or experimental model of Chagas disease. In this way, angiotensin converting enzyme inhibition and β-blockers, prescribed routinely to Chagasic patients, has already proven to be effective in reducing the occurrence of adverse events in inflammatory-dependent CC in human and experimental models.23–26
Data from this present study show that therapy with low doses of Simvastatin, the inhibitor of HMG-CoA reductase, significantly retards the progression of CC in dogs, which is the well-defined model to study immunological and clinical responses of Chagas disease because of its similarity with the human data.17 The key finding here is that Simvastatin administered to combat T. cruzi infection can modulate the immune response in hosts during initial steps of acute phase and persist during the chronic phase. Many of theses cholesterol-independent effects reflect the ability of statins to affect on this anti-inflammatory process (e.g., reducing activation of the transcription factor nuclear factor κB, modulating nitric oxide, endothelin-1, and pro-inflammatory cytokines, and blocking the synthesis of important isoprenoid intermediates).13,14 By inhibiting l-mevalonic acid synthesis, statins prevent the synthesis of other important isoprenoid intermediates of the cholesterol biosynthetic pathway, such as farnesylpyrophosphate and geranylgeranylpyrophosphate.27 In trypanosomatids, the importance of isoprenoids for cell viability and proliferation has previously been proven, and the combination of inhibitors that act at different points of the pathway seems to be useful against T. cruzi.28
However, we do not observe interference of low doses of Simvastatin on trypomastigote surviving during the acute phase. Conversely, there was an increased level of circulating parasites during 2 weeks post-infection, culminating in a higher parasitemia peak observed in those dogs treated with Simvastatin. These data could be partially explained by the gap failure in the inflammatory response. Pro-inflammatory cytokines (e.g., IFN-γ and TNF-α) have been shown to be essential to activate and drive macrophages in controlling parasites growth, dependently or not of nitric oxide.29,30 In addition to these activated macrophages, there is a clear role for CD4 and CD8 T cells in the control of the acute phase7 and also, in the magnitude of inflammatory response through chemokine leukocyte recruitment in vivo or in vitro.8,31 In accordance with these points, Simvastatin reduced serum levels of TNF-α and IFN-γ in all infected animals, and possibly, this modulation interfered directly with the control of circulating parasites during the acute phase. This pattern of pro-inflammatory cytokines was maintained by Simvastatin during the initial acute phase but was changed later by an increasing production of IL-10, not TNF-α and IFN-γ, coinciding with the beginning of the chronic phase. The regulatory cytokine IL-10 might interfere with the ability of the mammalian host to deal with the infection, especially controlling the Th1-predominant immune response against T. cruzi and possibly, preventing unwanted excessive heart inflammation.32,33
This Th polarization was also observed in the heart tissue (low mRNA expression for IFN-γ and high for IL-10) from those animals treated for 6 months with statins. Taking the similarity of dog models to human immunogenesis of Chagas disease, our data might suggest a protective condition against the inflammatory process induced by Simvastatin and consequently, for CC remodeling. Gomes and others32 show the importance of Th cytokine polarization to the prognosis of human CC. Their data, supported by others, can be explained by the abilities of pro-inflammatory cytokines (e.g., IFN-γ) to modulate chemokine production, which is responsible for driving leukocyte recruitment to eliminate antigenic stimuli in the inflammatory site.32,33 This inflammatory response, considered necessary for parasite control, can become unwanted, provoking tissue destruction, fibrosis, and consequently, cardiac remodeling with loss of contraction and functionality. Another important point is that, even when interfering in the blood parasite replication during acute phase, the chronic therapy of Simvastatin was able to modulate the inflammatory response without inducing a global immunosuppressive state in the dog and consequently, did not alter parasite replication in the chronic phase. Here, this was clearly observed by the unchanged number of amastigote nests in heart tissue in treated and untreated infected dogs.
Reinforcing this hypothesis, Simvastatin was able to significantly reduce the inflammatory infiltration in dogs infected with T. cruzi in both the right atrium and left ventricle, both chambers previously shown to be important targets of inflammation and fibrosis in the canine model of Chagas disease.34 In theory, these inflammatory aspects might then pre-dispose to myocyte slippage and remodeling of these chambers, especially in the left ventricle.35 In accordance, Simvastatin therapy also induced low serum levels and cardiac mRNA expression of pro-inflammatory cytokines (TNF-α and IFN-γ) during the recent chronic phase in our infected mongrel dogs. This immune response pattern was associated with a drastic reduction of inflammatory infiltration but not fibrosis at 6 months post-infection. It has been described that TNF-α can induce myocardial fibrosis by inhibiting collagen phagocytosis in the heart36 and up-regulating angiotensin II receptors on cardiac fibroblasts, leading to enhancement of the effects of its correlated hormone.37 In our present data, we did not correlate both inflammatory infiltration and quantification of collagen (fibrosis), because this collagen is probably a consequence of a previous inflammation that occurred during the acute phase. Using this hypothesis, less collagen would be expected in the future (more than 6 months of infection) for those animals treated with Simvastatin that presented less inflammation at the time of euthanasia and consequently, less remodeling with a better cardiac hemodynamic.
To our knowledge, our study is the first to show the effectiveness of Simvastatin in modulating pro-inflammatory and regulatory cytokines during CC and exerting an important role in the structural and functional parameter of remodeling. Because statins are commonly used in clinical practice with limited and well-recognized adverse effects, these drugs may be considered as possible adjuncts in the treatment of human CC.
ACKNOWLEDGMENTS
We recognize the financial support of Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG – Edital 01/2009, Processo APQ-00935-09), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Universidade Federal de Ouro Preto (UFOP), Rede Mineira de Biotérios and Laboratório Sanval (São Paulo, SP).
Footnotes
Authors' addresses: Lilian Melo, Ivo Santana Caldas, Maíra Araújo Azevedo, Vivian Paulino Figueiredo, Lívia de Figueiredo Diniz, Wanderson Geraldo de Lima, Maria Terezinha Bahia, and André Talvani, Núcleo de Pesquisas em Ciências Biológicas, Universidade Federal de Ouro Preto, Ouro Preto, MG, Brazil, E-mails: lilianmel@yahoo.com.br, ivocaldass@yahoo.com.br, mairaaazevedo@hotmail.com, vivian_figueiredo@yahoo.com.br, liviafcd@yahoo.com.br, wanderson@ufop.br, mtbahia@nupeb.ufop.br, and talvani@nupeb.ufop.br. Karolina Ribeiro Gonçalves, Alvaro Fernando da Silva do Nascimento, Wanderson Geraldo de Lima, and Maria Terezinha Bahia, Departamento de Ciências Biológicas, Universidade Federal de Ouro Preto, Ouro Preto, MG, Brazil, E-mails: krgbio@gmail.com, neto1988@gmail.com, wanderson@ufop.br, and mtbahia@nupeb.ufop.br. Rosália Moraes Torres, Faculdade de Medicina, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil, E-mail: rmtorres@medicina.ufmg.br.
References
- 1.Pan American Health Organization (PAHO)–World Health Organization (WHO) Estimacion cuantitativa de la enfermedad de Chagas en las Americas. Montevideo, Uruguay: Organizacion Panamericana de la Salud; 2006. CD/425-06. [Google Scholar]
- 2.Feldman AM, McNemara D. Myocarditis. N Engl J Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 3.Rocha MO, Teixeira MM, Ribeiro AL. An update on the management of Chagas cardiomyopathy. Expert Rev Anti Infect Ther. 2007;5:727–743. doi: 10.1586/14787210.5.4.727. [DOI] [PubMed] [Google Scholar]
- 4.Dutra WO, Rocha MO, Teixeira MM. The clinical immunology of human Chagas disease. Trends Parasitol. 2005;21:581–587. doi: 10.1016/j.pt.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez FR, Guedes PM, Gazzinelli RT, Silva JS. The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite Immunol. 2009;31:673–685. doi: 10.1111/j.1365-3024.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 6.Abrahamsohn IA, Coffman RL. Trypanosoma cruzi: IL-10, TNF, IFN-gamma and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol. 1996;84:231–244. doi: 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 7.Brener Z, Gazzinelli RT. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas disease. Int Arch Allergy Immunol. 1997;114:103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- 8.Talvani A, Ribeiro CS, Aliberti JC, Michailowsky V, Santos PV, Murta SM, Romanha AJ, Almeida IC, Farber J, Lannes-Vieira J, Silva JS, Gazzinelli RT. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-gamma as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes Infect. 2000;2:851–866. doi: 10.1016/s1286-4579(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 9.Urbina JA. New advances in the management of a long-neglected disease. Clin Infect Dis. 2009;49:1685–1687. doi: 10.1086/648073. [DOI] [PubMed] [Google Scholar]
- 10.Node K, Fujita M, Kitakaze M, Hori M, Liao JK. Short-term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathy. Circulation. 2003;108:839–843. doi: 10.1161/01.CIR.0000084539.58092.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanes JG, Minadeo KN, Moret A, Groner M, Tabaic SA. Statin therapy in heart failure: prognostic effects and potential mechanisms. Am Heart J. 2007;154:617–623. doi: 10.1016/j.ahj.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 13.Cheng X, Liao YH, Zhang J, Li B, Ge H, Yuan J, Wang M, Lu B, Liu Y, Cheng Y. Effects of Atorvastatin on Th polarization in patients with acute myocardial infarction. Eur J Heart Fail. 2005;7:1099–1104. doi: 10.1016/j.ejheart.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Robinson JG. Models for describing relations among the various statin drugs, low-density lipoprotein cholesterol lowering, pleiotropic effects and cardiovascular risk. Am J Cardiol. 2008;101:1009–1015. doi: 10.1016/j.amjcard.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 15.Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, DeMaria AN. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 16.Mancini GB, Etminan M, Zhang B, Levesque LE, FitzGerald JM, Brophy JM. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006;47:2554–2560. doi: 10.1016/j.jacc.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 17.Guedes PM, Veloso VM, Afonso LC, Caliari MV, Carneiro CM, Diniz LF, Marques-da-Silva EA, Caldas IS, Do Valle Matta MA, Souza SM, Lana M, Chiari E, Galvão LM, Bahia MT. Development of chronic cardiomyopathy in canine Chagas disease correlates with high IFN-gamma, TNF-alpha, and low IL-10 production during the acute infection phase. Vet Immunol Immunopathol. 2009;130:43–52. doi: 10.1016/j.vetimm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Guedes PM, Veloso VM, Talvani A, Diniz LF, Caldas IS, Do-Valle-Matta MA, Santiago-Silva J, Chiari E, Galvão LM, Silva JS, Bahia MT. Increased type 1 chemokine expression in experimental Chagas disease correlates with cardiac pathology in Beagle dogs. Vet Immunol Immunopathol. 2010;138:106–113. doi: 10.1016/j.vetimm.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Zaca V, Rastogi S, Imai M, Wang M, Sharov VG, Jiang A, Goldstein S, Sabbah HN. Chronic monotherapy with Rosuvastatin prevents progressive left ventricular dysfunction and remodeling in dogs with heart failure. J Am Coll Cardiol. 2007;50:551–557. doi: 10.1016/j.jacc.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 20.Shiroshita-Takeshita A, Brundel BJJM, Burstein B, Leung TK, Mitamura H, Ogawa S, Nattel S. Effects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failure. Cardiovasc Res. 2007;74:75–84. doi: 10.1016/j.cardiores.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen TR, Tobert JA. Simvastatin: a review. Expert Opin Pharmacother. 2004;5:2583–2596. doi: 10.1517/14656566.5.12.2583. [DOI] [PubMed] [Google Scholar]
- 22.Morris SA, Tanowitz HB, Wittner M, Bilezikian JP. Pathophysiological insights into the cardiomyopathy of Chagas disease. Circulation. 1990;82:1900–1909. doi: 10.1161/01.cir.82.6.1900. [DOI] [PubMed] [Google Scholar]
- 23.Botoni FA, Poole-Wilson PA, Ribeiro AL, Okonko DO, Oliveira BM, Pinto AS, Teixeira MM, Teixeira AL, Jr, Reis AM, Dantas JB, Ferreira CS, Tavares WC, Jr, Rocha MO. A randomized trial of carvedilol after renin-angiotensin system inhibition in chronic Chagas cardiomyopathy. Am Heart J. 2007;153:544e1–544e8. doi: 10.1016/j.ahj.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Bestetti RB, Theodoropoulos TA, Cardinalli-Neto A. Treating patients with Chagas cardiomyopathy with chronic heart failure in the contemporary era. Am Heart J. 2007;154:35e. doi: 10.1016/j.ahj.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 25.Issa VS, Amaral AF, Cruz FD, Ferreira SM, Guimarães GV, Chizzola PR, Souza GE, Bacal F, Bocchi EA. Beta-Blocker therapy and mortality of patients with Chagas cardiomyopathy: a subanalysis of the REMADHE prospective Trial. Circ Heart Fail. 2010;3:82–88. doi: 10.1161/CIRCHEARTFAILURE.109.882035. [DOI] [PubMed] [Google Scholar]
- 26.Paula-Costa G GP, Silva RR, Pedrosa MC, Pinho V, Lima WG, Teixeira MM, Bahia MT, Talvani A. Enalapril prevents cardiac immune-mediated damage and exerts anti-Trypanosoma cruzi activity during acute phase of experimental Chagas disease. Parasite Immunol. 2010;32:202–208. doi: 10.1111/j.1365-3024.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 28.Concepcion JL, Gonzalez-Pacanowska D, Urbina JA. 3-Hydroxy-3-methyl-glutaryl-CoA reductase in Trypanosoma (Schizotrypanum) cruzi: subcellular localization and kinetic properties. Arch Biochem Biophys. 1998;352:114–120. doi: 10.1006/abbi.1998.0577. [DOI] [PubMed] [Google Scholar]
- 29.Silva JS, Machado FS, Martins GA. The role of nitric oxide in the pathogenesis of Chagas disease. Front Biosci. 2003;1:314–325. doi: 10.2741/1012. [DOI] [PubMed] [Google Scholar]
- 30.Cummings KL, Tarleton RL. Inducible nitric oxide synthase is not essential for control of Trypanosoma cruzi infection in mice. Infect Immun. 2004;72:4081–4089. doi: 10.1128/IAI.72.7.4081-4089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coelho PS, Klein A, Talvani A, Coutinho SF, Takeuchi O, Akira S, Silva JS, Canizzaro H, Gazzinelli RT, Teixeira MM. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes induce in vivo leukocyte recruitment dependent on MCP-1 production by IFN-gamma-primed-macrophages. J Leukoc Biol. 2002;71:837–844. [PubMed] [Google Scholar]
- 32.Gomes JA, Bahia-Oliveira LM, Rocha MO, Martins-Filho OA, Gazzinelli G, Correa-Oliveira R. Evidence that development of severe cardiomyopathy in human Chagas' disease is due to a Th1-specific immune response. Infect Immun. 2003;71:1185–1193. doi: 10.1128/IAI.71.3.1185-1193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunha-Neto E, Nogueira LG, Teixeira PC, Ramasawmy R, Drigo SA, Goldberg AC, Fonseca SG, Bilate AM, Kalil J. Immunological and non-immunological effects of cytokines and chemokines in the pathogenesis of chronic Chagas disease cardiomyopathy. Mem Inst Oswaldo Cruz. 2009;104((Suppl 1)):252–258. doi: 10.1590/s0074-02762009000900032. [DOI] [PubMed] [Google Scholar]
- 34.de Lana M, Chiari E, Tafuri WL. Experimental Chagas' disease in dogs. Mem Inst Oswaldo Cruz. 1992;87:59–71. doi: 10.1590/s0074-02761992000100011. [DOI] [PubMed] [Google Scholar]
- 35.Gunja-Smith Z, Morales AR, Romanelli R, Woessner FR., Jr Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline crosslinks. Am J Pathol. 1996;1448:1639–1648. [PMC free article] [PubMed] [Google Scholar]
- 36.Chou DH, Lee W, McCulloch CA. TNF-alpha inactivation of collagen receptors: implications for fibroblasts function and fibrosis. J Immunol. 1996;156:4354–4362. [PubMed] [Google Scholar]
- 37.Gurantz D, Cowling RT, Villarreal FJ, Greenberg BH. Tumor necrosis factor-alpha upregulates angiotensin II type 1 receptors on cardiac fibroblasts. Circ Res. 1999;85:272–279. doi: 10.1161/01.res.85.3.272. [DOI] [PubMed] [Google Scholar]


