Abstract
Pregnancy in women with lupus nephritis is associated with increased risk of fetal and maternal complications. The risk of poor outcome is higher if there are signs of disease activity at conception. The presence of hypertension and anti-phospholipid antibodies worsens the prognosis. There are very few therapeutic options in view of the threat of various congenital anomalies and associated comorbidities. Mycofenolate mofetil (MMF) is contraindicated during pregnancy due to risk of congenital anomalies and fetal loss. This is a case of a woman with membranous lupus nephritis, who went into partial remission with rituximab and became pregnant while on maintenance therapy with MMF. Due to lack of alternative options, she continued to be given MMF. She had a successful outcome in spite of the presence of the poor prognostic factors. The baby had asymptomatic non-communicating duplication of the oesophagus, which has never been reported before in association with MMF during pregnancy.
BACKGROUND
Treatment of lupus nephritis during pregnancy is challenging. Mycofenolate mofetil (MMF) is contraindicated during pregnancy and it has been classed as category D (positive evidence of fetal risk) by the US Food and Drug Administration (FDA). This case illustrates an example of a rare clinical situation where its use can be justified. In spite of the presence of a number of poor prognostic markers such as proteinuria, hypertension and the presence of lupus anticoagulant this patient had successful outcome. The baby was noted to have an asymptomatic congenital anomaly that has never been described before, with MMF use in pregnancy.
CASE PRESENTATION
A 21-year-old Caucasian woman presented in 2001 with fatigue, myalgia, arthralgia and a malar rash. Investigations revealed pancytopoenia. Her haemoglobin (Hb) was 9.2 g/dl, white cell count (WCC) was 1.9×109 cells/dl, platelets were 53×109 cells/dl and her erythrocyte sedimentation rate (ESR) was 107 mm/h. She had nephrotic range proteinuria of 4 g/24 h, serum albumin of 33 g/dl and serum creatinine of 56 μmol/litre. She was normotensive. Lupus serology was positive in the form of elevated anti-nuclear antibody (ANA) (>1:640), anti-double-stranded DNA (dsDNA) (1125 IU/ml) and a weakly positive lupus anticoagulant. The diagnosis of membranous nephropathy was confirmed on renal biopsy. She was given corticosteroids and azathioprine. She developed severe exfoliating dermopathy with azathioprine. Subsequently she was given MMF and corticosteroid combination. Hydroxychloroquine was added to help her musculoskeletal symptoms. Her symptoms improved to some extent but persisted in a milder form. Her haematological parameters normalised but her autoantibodies remained significantly positive and she continued to have ongoing proteinuria with normal renal function. She relapsed in July 2003 with worsening constitutional symptoms, arthralgia and pleuritic chest pain and her haematological parameters worsened in the form of pancytopoenia. She was switched to cyclophosphamide at a dose of 0.75 g/m2 (1 g) every 4 weeks, which was increased after the third dose to 1.5 g/m2 every 3 weeks due to worsening symptoms. Prednisolone was continued. After the seventh dose of cyclophosphamide in December 2003 she developed more severe symptoms, her lupus serology remained strongly positive and her proteinuria worsened from 4 g/24 h to 6.6 g/24 h. In January 2004 she was treated with rituximab at a dose of 375 mg/m2 intravenously, weekly, for 4 weeks. Her haematological parameters significantly improved but her symptoms had improved only partially, she had ongoing nephrotic range proteinuria with persistently positive autoantibodies and hence it was decided to continue her cyclophosphamide at a dose of 1 g every 4 weeks until July 2004. In August 2004, she became completely asymptomatic and her haematological parameters normalised. Her proteinuria reduced to 2.9 g/24 h. Her serum creatinine was 69 μmol/litre and serum albumin was 38 g/litre. Her immunological markers improved but remained positive at titres of ANA 1:320 and anti-dsDNA 524 IU/ml. In August 2004 she was switched to MMF 1 g twice daily as maintenance therapy and continued on prednisolone 7.5 mg per day along with losartan and bendroflumethazide for her hypertension. Over the next 3 years, her haematological and biochemical profile remained normal but her autoantibodies persisted and fluctuated with significant titres. She also continued to have significant proteinuria with normal creatinine. She was given oral contraceptives and was made aware of the risks of pregnancy while on the above medication. She was found to be 13 weeks pregnant in February 2008. Risks including congenital malformation due to exposure to MMF during early pregnancy were explained. The patient was reluctant to consider termination. In view of significant risk of relapse of lupus if MMF is withdrawn and the fact that there was no suitable alternative immunosuppressant during pregnancy in the event of relapse, a decision was made to continue with MMF and prednisolone. Losartan and bendrofluazide were withdrawn and her hypertension was controlled with nifedepine and methyldopa. Aspirin and low molecular weight heparin therapy was initiated in view of the presence of lupus anticoagulant. An ultrasound anomaly scan at the 20th week of gestation detected a cystic structure in the oesophagus of the fetus, the significance of which was uncertain at that time. She continued to have around 3 g proteinuria per day throughout pregnancy. The blood pressure control was excellent with normal renal excretory function and serum albumin. Her haematological parameters remained normal throughout pregnancy. There was no evidence of pre-eclampsia.
OUTCOME AND FOLLOW-UP
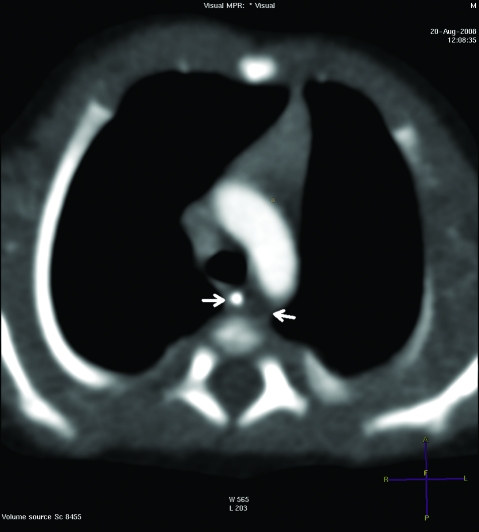
She delivered a baby girl by lower segment Caesarean section at 35 weeks due to failure of progression of breech presentation. The baby girl was well at birth, but after 1 week she became profoundly septic, developed possible necrotising enterocolitis and was transferred to a tertiary care centre. She grew Acinobacter from her blood cultures and was treated with appropriate antibiotics. Necrotising enterocolitis was conservatively managed with total parenteral nutrition. The cystic structure adjacent to her oesophagus found on the prenatal anomaly scan was confirmed to be a non-communicating duplication of the oesophagus measuring 1.5×0.5×0.5 cm on a contrast enhanced CT of the chest (fig 1). The baby improved with the intensive treatment and was discharged home after 3 weeks. The baby has continued to do well at 6 months of age and remained asymptomatic from her oesophageal duplication. The mother has remained well but she continues to have ongoing proteinuria and hypertension.
Figure 1.
Non-communicating duplication of the oesophagus noted on CT of the chest (between arrows).
DISCUSSION
Pregnancy in women with lupus nephritis is associated with an increased risk of fetal and maternal complications. It is uncertain whether pregnancy exacerbates lupus, but in pregnancies in which lupus is clinically active at conception, the risk of relapse is high and levels up to 61% have been quoted in the literature.1 It is not clear, however, if this disease flares due to pregnancy per se. The risk of poor maternal and fetal outcome is much higher if there are signs of activity, which include proteinuria >0.5 g/day.2 Fetal loss of up to 38% has been reported in patients with lupus with prepregnant proteinuria of >0.5 g/day. In patients with lupus nephritis, the presence of pre-existing hypertension as well as anti-phospholipid antibodies are significant risk factors for pre-eclampsia with its associated morbidity and mortality. In spite of the presence of all these features, our patient had a successful outcome.
Postmarketing data of MMF from the US National Transplantation Pregnancy Registry has shown increased risk of first trimester pregnancy loss and congenital malformation. Manufacturer’s data collected from 1995 to 2007 on 77 women exposed to MMF during pregnancy showed that 25 had spontaneous abortions and 14 had malformed infants.3 The category for MMF during pregnancy has been changed from category C (risk of fetal harm cannot be ruled out) to category D (positive evidence of fetal risk) by the US FDA. The decision to continue MMF in the above case was made due to lack of alternative options. There was a significant risk of relapse if MMF was withdrawn and not replaced with an alternative immunosuppressant. The patient was previously intolerant of azathioprine. Cyclophosphamide is considered a category D drug in terms of its risk for pregnancy by the FDA. Even though she responded partially to rituximab in the past, the experience in pregnancy in the literature is limited to three case reports, hence the use of rituximab is not recommended in pregnancy in the event of relapse.4–6 The patient refused to accept termination of pregnancy as an option. Given the fact that the fetus was already exposed to MMF for 13 weeks, continuing with MMF and steroid was considered the best option after obtaining the patient’s informed consent. During the neonatal period, the baby experienced a septic episode. It is logical to infer that this episode could possibly be secondary to transient immune alteration in the infant after MMF exposure all through out the pregnancy. We conclude that MMF had a role to play in the development of severe septicaemia in the neonate even though it cannot be proven.
The current literature suggests that congenital anomalies can occur in up to 23% of the babies exposed to MMF during pregnancy.7 In utero exposure to MMF can cause a characteristic phenotype whose main features are orofacial and auditory developmental anomalies including cleft lip and palate, bilateral anotia, microtia with atresia of external auditory canal, micrognathia, polydactylia, nail hypoplasia and hypertelorism.8,9 Other visceral anomalies that have been reported include agenesis of the corpus callosum, left pelvic ectopic kidney, asymmetry of the kidneys, congenital diaphragmatic hernia, congenital heart defect (anterior positioning of the aorta, interventricular communication), and aberrant blood vessels between the trachea and oesophagus.8,9 A non-communicating duplication of the oesophagus has not been reported in the literature. Even though there is a theoretical possibility that losartan could be responsible for the congenital malformation, MMF is likely to be the culprit as it is more strongly associated with teratogenicity. Our case report contributes a new finding to the current wealth of information available on effects of MMF during pregnancy on fetal outcome. In conclusion, this case illustrates an example of an extremely challenging clinical situation with successful outcome.
LEARNING POINTS
Mycofenolate mofetil (MMF) is contraindicated in pregnancy due to positive evidence of fetal risk.
In clinical situations where the risk benefit analysis justifies its use, successful maternal and fetal outcome is possible.
A number of congenital anomalies have been described with MMF use in pregnancy, which possibly includes asymptomatic non-communicating duplication of the oesophagus.
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Bobrie G, Liote F, Houillier P, et al. Pregnancy in lupus nephritis and related disorders. Am J Kidney Dis 1987; 9: 339–43 [DOI] [PubMed] [Google Scholar]
- 2.Kong CTN. Pregnancy of a lupus patient - a challenge to the nephrologist. Nephrol Dial Transplant 2006; 21: 268–72 [DOI] [PubMed] [Google Scholar]
- 3.Roche USA http://www.rocheusa.com/products/cellcept/pregnancy_notice.pdf (accessed 18 September 2008) [Google Scholar]
- 4.Friedrichs B, Tiemann M, Salwender H, et al. The effects of rituximab treatment during pregnancy on a neonate. Haematologica 2006; 91: 1426–7 [PubMed] [Google Scholar]
- 5.Herold M, Schnohr S, Bittrich H. Efficacy and safety of a combined rituximab chemotherapy during pregnancy. J Clin Oncol 2001; 19: 3439. [PubMed] [Google Scholar]
- 6.Kimby E, Sverrisdottir A, Elinder G. Safety of rituximab therapy during the first trimester of pregnancy: a case history. Eur J Haematol 2004; 72: 292–5 [DOI] [PubMed] [Google Scholar]
- 7.Ostensen M, Khamashta M, Lockshin M, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther 2006; 8: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez–Aytes A, Ledo A, Boso V, et al. In utero exposure to mycophenolate mofetil: a characteristic phenotype. Am J Med Genet 2008; 146: 1–7 [DOI] [PubMed] [Google Scholar]
- 9.Sebaaly ZE, Charpentier B, Snanoudi R. Fetal malformations associated with mycophenolate mofetil for lupus nephritis. Nephrol Dial Transplant 2007; 22: 2722. [DOI] [PubMed] [Google Scholar]