Abstract
Purpose
The purpose of this study was to clarify the prognostic significance of lymphocyte infiltration in soft tissue sarcomas (STS). Prognostic markers in potentially curable STS should guide therapy after surgical resection. The immune status at the time of resection may be important, but the prognostic significance of tumor infiltrating lymphocytes is controversial as the immune system has conflicting roles during cancer development.
Experimental Design
Tissue microarrays from 249 patients with STS were constructed from duplicate cores of viable and representative neoplastic tumor areas. Immunohistochemistry was used to evaluate the CD3+, CD4+, CD8+, CD20+ and CD45+ lymphocytes in tumors.
Results
In univariate analyses, increased numbers of CD4+ (P = 0.008) and CD20+ (P = 0.006) lymphocytes in tumor correlated significantly with an improved disease-specific survival (DSS) in patients with wide resection margins (n = 108). In patients with non-wide resection margins (n = 141) increased numbers of CD3+ (P = 0.028) lymphocytes in tumor correlated significantly with shorter DSS. In multivariate analyses, a high number of CD20+ lymphocytes (HR = 5.5, CI 95% = 1.6–18.6, P = 0.006) in the tumor was an independent positive prognostic factor for DSS in patients with wide resections margins.
Conclusions
High density of CD20+ lymphocytes in STS with wide resection margins is an independent positive prognostic indicator for these patients. Further research is needed to define if CD20+ cells can modify tumors in a way that reduces disease progression and metastatic potential.
Introduction
Soft tissue sarcomas (STS) are relatively rare, heterogeneous malignancies of mesenchymal origin with a high mortality rate. They comprise less than 1% of adult malignancies[1] and approximately 50% of the STS patients will succumb to their disease because of metastasis or local relapse[2]. There are several prognostic factors which determine tumour progression, and ultimately the patient's outcome, including positive resection margins; presence of local recurrence; and tumour grade, size, location, depth and histological entity[3]–[9].
Many studies have been designed to investigate the prognostic factors of STS by using immuno-histochemical methods[10]. Most of the published data have focused on the expression of markers for cell kinetics and regulatory proteins of the cell cycle.
Tumor infiltrating lymphocytes are considered to be an indication of the host immune reaction to tumor antigens[11], and their clinical significance has been reported in a variety of human solid tumors.
CD3 is a part of the T cell receptor (TCR) complex on a mature T lymphocyte. CD4 is a glycoprotein expressed on the surface of T helper cells, regulatory T cells, monocytes, macrophages, and dendritic cells. CD8 is a transmembrane glycoprotein that serves as a co-receptor for the T cell receptor (TCR). Like the TCR, CD8 binds to a major histocompatibility complex (MHC) molecule, but is specific for the class I MHC protein. CD20 is a non-glycosylated phosphoprotein expressed on the surface of all mature B-cells. CD20 is expressed on all stages of B cell development except the first and last; it is present from pre-pre B cells through memory cells, but not on either pro-B cells or plasma cells. The CD45 antigen was originally called leukocyte common antigen. The protein encoded by this gene is a member of the protein tyrosine phosphatase (PTP) family. This gene is specifically expressed in hematopoietic cells. This PTP has been shown to be an essential regulator of T- and B-cell antigen receptor signalling (http://www.genecards.org).
The purpose of this study was to clarify the prognostic significance of lymphocyte infiltration in non-gastrointestinal stromal tumor (GIST) STSs. To achieve this, we analyzed the expression of CD3+, CD4+, CD8+, CD20+ and CD45+ lymphocytes in 249 patients with non-GIST STS in relation to other clinicopathological variables.
Materials and Methods
Patients and Clinical Samples
The National Cancer Data Inspection Board and The Regional Committee for Research Ethics approved the study. The Regional Committee approved that written consent from the patients for their information to be stored in the hospital database and used for research was not needed. This because most of the material was more than 10 years old, and most of the patients being dead. The material was collected from our approved biobank for paraffin embedded material and slides. Data were analyzed anonymously.
Primary tumor tissues from patients diagnosed with STS at the University Hospital of North Norway (UNN) from 1973 to 2006 and the Hospitals of Arkhangelsk region, Russia, were used in this retrospective study. 496 potentially suitable patient records were identified from the hospital database but only 249 of these were eligible for this study because they had complete medical records and adequate paraffin-embedded tissues blocks. This report includes follow-up data for 167 Norwegian patients and 82 Russian patients up to September 2009. The median follow-up was 38 (range 0–392) months. Complete demographic and clinical data were collected retrospectively. Formalin-fixed and paraffin-embedded tumor specimens were obtained from the archives of the Departments of Pathology at UNN and Archangelsk. The tumors were graded according to the French Fèdèration Nationales des Centres de Lutte Contre le Cancer (FNCLCC), [WHO Tumors of Soft Tissue and bone, 2002]. Wide resection margins were defined as wide local resection with free microscopic margins or amputation of the affected limb or organ. Non-wide resection margins were defined as either marginal or intralesional resection margins, or no surgery.
Microarray construction
The histology of all soft tissue sarcoma cases were reviewed by two pathologists (AV and SWS). Tissue microarrays (TMAs) were constructed for high-throughput molecular pathology research[12]. The most representative areas of viable tumor cells were carefully selected and marked on the hematoxylin and eosin (HE) slides for the corresponding donor blocks and sampled for the tissue microarray collector blocks. The TMAs were assembled using a tissue-arraying instrument (Beecher Instruments).
Studies suggest that punching multiple 0.6 mm cores from different regions captures the heterogeneity of the tumors more accurately than single 2 to 4 mm core[13]. Hence, we chose using two 0.6-mm cores of viable neoplastic tissue that were selected to be as representative as possible (different areas), after reviewing all original sections of the tumor and taking the heterogeneity in consideration. To include all core samples, 12 tissue array blocks were constructed. Multiple 4-µm sections were cut with a Micron microtome (HM355S) and stained by specific antibodies for immunohistochemistry (IHC).
Immunohistochemistry (IHC)
The applied antibodies were subjected to in-house validation by the manufacturer for IHC analysis on paraffin-embedded material. Ventana Benchmark, XT automated slide stainer (Ventana Medical System, France) was used for IHC. Sections were deparaffinized with xylene and rehydrated with ethanol. Antigen retrieval was performed by placing the specimens in 0.01 M citrate buffer at pH 6.0 and exposing them to two repeated microwave heatings of 10 minutes each at 450W. The DAKO Envision+ System-HRP (DAB) kit was used as endogen peroxidase blocking. As negative staining controls, the primary antibodies were replaced with the primary antibody diluents. Primary mouse monoclonal antibodies were incubated for 16 minutes (CD20, clone L26 Ventana), 20 minutes (CD4, clone 1F6 Novocastra, dilution 1∶5) and 32 minutes (CD8, clone 1A5 Ventana) at room temperature. The Ventana antibodies were pre-diluted from the manufacturer. Biotinylated goat anti-mouse IgG and mouse anti-rabbit IgM were used as secondary antibodies. The DAB was used to visualize the antigens. This was followed by application of liquid diaminobenzidine and substrate-chromogen, yielding a brown reaction product at the site of the target antigen. Finally, slides were counterstained with hematoxylin to visualize the nuclei. For each antibody, including negative controls, all TMA staining was performed in a single experiment.
Scoring of IHC
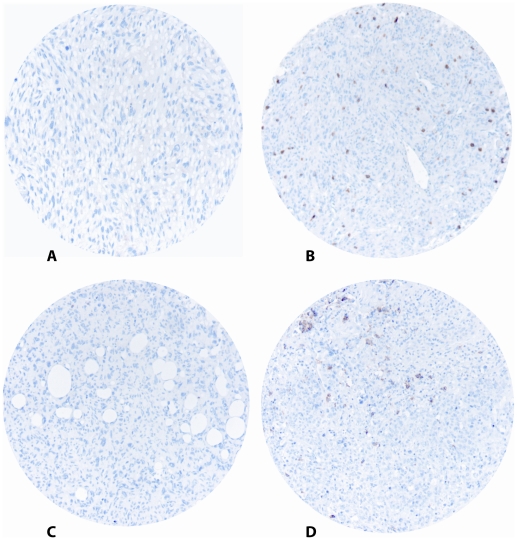
The ARIOL imaging system (Genetix, San Jose, CA) was used to scan the slides for antibody staining of the TMAs. The specimens were scanned at a low resolution (1.25×) and high resolution (20×) using an Olympus BX 61 microscope with an automated platform (Prior). The slides were loaded in the automated slide loader (Applied Imaging SL 50). Representative and viable tissue sections were scored manually on a computer screen semi-quantitatively for cytoplasmic staining. Tumors were scored as 0 (no cells), 1 (1–5 cells), 2 (6–19 cells) or 3 (20+ cells) (Figure 1). All samples were made anonymous and independently scored by two pathologists (AV and SWS). Where there was disagreement, the slides were re-examined and a consensus was reached by the observers. When assessing a variable for a given score, the scores of the other variables and the outcome were hidden from the observers.
Figure 1. IHC microscopic pictures of TMA of soft tissue sarcoma representing different scores for CD4+ and CD20+ lymphocytes.
(A) CD4 low score; (B) CD4 high score; (C) CD20 low score; (D) CD20 high score. Original magnification X 400.
Statistical Methods
All statistical analyses were done using the statistical package SPSS (Chicago, IL), version 16. The immunohistochemistry scores from each observer were compared for interobserver reliability by use of a two-way random effect model with absolute agreement definition. The intraclass correlation coefficient (reliability coefficient) was obtained from these results.
The Chi-square test and Fishers Exact test were used to examine the association between molecular marker expression and various clinicopathological parameters. Univariate analyses were done using the Kaplan-Meier method, and statistical significance between survival curves was assessed by the log rank test. Disease-specific survival (DSS) was determined from the date of histological-confirmed STS diagnosis.
Multivariate analysis was carried out using the Cox proportional hazards model to assess the specific impact of each pre-treatment variable on survival in the presence of other variables. Only variables of significant value from the univariate analysis were entered into the Cox regression analysis. Probability for stepwise entry and removal was set at 0.05 and 0.10, respectively. The significance level used was p<0.05.
Results
Clinicopathological Variables
Demographic, clinical, and histopathological variables are shown in Table 1. Patient age range was 0–91 years (mean 55 years), and 44% of the patients were males. The non-GIST STS comprised 68 undifferentiated pleomorphic sarcoma, 67 leiomyosarcoma, 34 liposarcoma, 20 malignant fibroblastic/myofibroblastic tumors, 16 rhabdomyosarcoma, 16 synovial sarcoma, 13 angiosarcoma, 11 malignant peripheral nerve sheath tumors (MPNST) and 4 other STS. There were 61 low grade STS (24%) and 188 high grade (FNCLCC grade 2 and 3) STS (76%).
Table 1. Prognostic clinicopathologic variables as predictors for disease-specific survival soft tissue sarcomas (univariate analysis, log rank test), N = 249.
| Characteristic | Patients(n) | Patients(%) | Median survival(months) | 5-Year survival(%) | P | |
| Age | ||||||
| ≤20 years | 20 | 8 | 15 | 40 | 0.126 | |
| 21–60 years | 113 | 45 | 68 | 52 | ||
| >60 years | 116 | 47 | 30 | 40 | ||
| Gender | ||||||
| Male | 110 | 44 | 41 | 46 | 0.390 | |
| Female | 139 | 56 | 45 | 45 | ||
| Nationality | ||||||
| Norwegian | 167 | 67 | 63 | 51 | 0.011 | |
| Russian | 82 | 33 | 22 | 34 | ||
| Histology | ||||||
| Undifferentiatedpleomorphic sarcoma | 68 | 27 | 29 | 40 | 0.102 | |
| Leiomyosarcoma | 67 | 27 | 45 | 46 | ||
| Liposarcoma | 34 | 14 | NR | 67 | ||
| MF/MFT | 20 | 8 | 43 | 50 | ||
| Angiosarcoma | 13 | 5 | 10 | 31 | ||
| Rhabdomyosarcoma | 16 | 6 | 17 | 38 | ||
| MPNST | 11 | 4 | 49 | 45 | ||
| Synovial sarcoma | 16 | 6 | 31 | 29 | ||
| Other STS | 4 | 2 | NR | 75 | ||
| Tumor localization | ||||||
| Extremities | 89 | 36 | 100 | 53 | 0.348 | |
| Trunk | 47 | 29 | 32 | 44 | ||
| Retroperitoneum | 37 | 25 | 25 | 38 | ||
| Head/Neck | 18 | 7 | 15 | 41 | ||
| Visceral | 58 | 23 | 30 | 42 | ||
| Tumor size | ||||||
| ≤5 cm | 74 | 30 | 127 | 57 | 0.027 | |
| 5–10 cm | 91 | 37 | 44 | 45 | ||
| >10 cm | 81 | 32 | 28 | 37 | ||
| Missing | 3 | 1 | ||||
| Malignancy grade FNCLCC | ||||||
| 1 | 61 | 25 | NR | 74 | <0.001 | |
| 2 | 98 | 39 | 41 | 45 | ||
| 3 | 90 | 36 | 16 | 26 | ||
| Tumor depth | ||||||
| Superficial | 17 | 7 | NR | 93 | <0.001 | |
| Deep | 232 | 93 | 36 | 42 | ||
| Metastasis at time of diagnosis | ||||||
| No | 206 | 83 | 76 | 53 | <0.001 | |
| Yes | 43 | 17 | 10 | 10 | ||
| Surgery | ||||||
| Yes | 228 | 92 | 59 | 50 | <0.001 | |
| No | 21 | 8 | 5 | 0 | ||
| Surgical margins | ||||||
| Wide | 108 | 43 | NR | 62 | <0.001 | |
| Non-wide | 141 | 57 | 19 | 33 | ||
| Chemotherapy | ||||||
| No | 191 | 77 | 52 | 47 | 0.424 | |
| Yes | 58 | 23 | 29 | 40 | ||
| Radiotherapy | ||||||
| No | 176 | 71 | 48 | 46 | 0.590 | |
| Yes | 73 | 29 | 38 | 43 | ||
Abbreviations: MF/MFT, malignant fibroblastic/myofibroblastic tumors; MPNST, malignant peripheral nerve sheath tumor; STS, soft tissue sarcomas; NR, not reached; NOS, non specified.
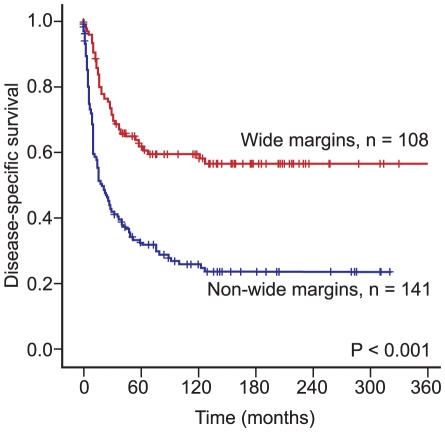
The treatment option of choice was surgery (n = 228): 118 patients received surgery only; 55 patients received surgery and radiotherapy; 40 patients received surgery and chemotherapy; 13 patients received surgery, radiotherapy and chemotherapy; 2 patients received chemotherapy only; 3 patients received chemotherapy and radiotherapy; 2 patients received radiotherapy only; and16 patients received no therapy. The 5-year survival with non-wide resection margins was 33% and with wide resection margins it was 62%.
Inter-observer variability
There was good reproducibility between the two investigating pathologists. Their scoring agreement was tested for CD8 and CD20. The IHC scores from each observer were compared using a two-way random effect model with absolute agreement definition. The intra-class correlation coefficients (reliability coefficients, r) obtained from these results were 0.90 for CD8 (P<0.001) and 0.90 for CD20 (P<0.001).
Univariate analyses
Nationality, tumor size, malignancy grade, tumor depth, metastasis at time of diagnosis, surgery and surgical margins were all significant indicators for disease-specific survival (DSS) in univariate analyses (Table 1, Figure 2). Most of the patients with non-GIST STS who did not survive their disease, died within the first 10 years (120 months). After 10 years almost 60% (n = 108) of the patients with wide resection margins were alive, but only 20% (n = 141) of patients with non-wide resection margins or no surgery (P<0.001), (Figure 2).
Figure 2. Disease-specific survival curves for patients with wide resection margins compared to patients with non-wide resection margins.
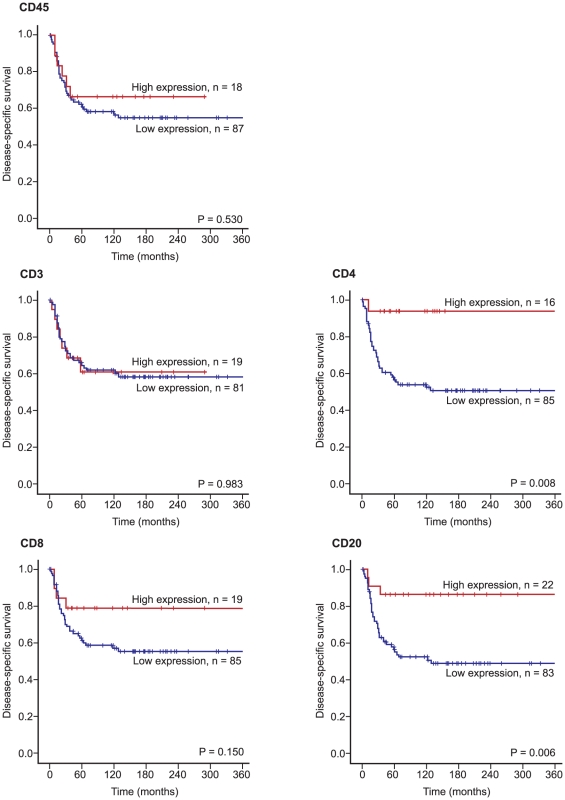
Furthermore, increasing numbers of CD4+ (P = 0.008) and CD20+ lymphocytes in tumor (P = 0.006) correlated significantly with an improved DSS in patients with wide resection margins (n = 108), (Table 2 and Figure 3). No such relationship was apparent for CD3+, CD8+ and CD45+ lymphocytes. In patients with non-wide resection margins (n = 141) increasing numbers of CD3+ lymphocytes correlated significantly (P = 0.028) with shorter DSS, (Table 2).
Table 2. Intratumoral lymphocyte infiltration and their prediction for disease-specific survival in patients with soft tissue sarcomas (univariate analysis; log-rank test), N = 249.
| Non-wide resections margins, n = 141 | Wide resection margins, n = 108 | |||||||||
| Marker expression | Patients(n) | Patients(%) | Median survival(months) | 5-Year survival(%) | P | Patients(n) | Patients(%) | Median survival(months) | 5-Year survival(%) | P |
| CD 3 | ||||||||||
| Low | 95 | 67 | 26 | 39 | 0.028 | 81 | 75 | NR | 64 | 0.983 |
| High | 26 | 18 | 15 | 28 | 19 | 18 | NR | 61 | ||
| Missing | 20 | 14 | 8 | 7 | ||||||
| CD 4 | ||||||||||
| Low | 112 | 79 | 23 | 35 | 0.474 | 85 | 79 | NR | 57 | 0.008 |
| High | 18 | 13 | 12 | 33 | 16 | 15 | NR | 94 | ||
| Missing | 11 | 8 | 7 | 6 | ||||||
| CD 8 | ||||||||||
| Low | 98 | 70 | 22 | 37 | 0.349 | 85 | 79 | NR | 61 | 0.150 |
| High | 21 | 15 | 26 | 29 | 19 | 18 | NR | 79 | ||
| Missing | 22 | 16 | 4 | 4 | ||||||
| CD 20 | ||||||||||
| Low | 103 | 73 | 26 | 34 | 0.447 | 83 | 77 | NR | 55 | 0.006 |
| High | 20 | 14 | 11 | 35 | 22 | 20 | NR | 86 | ||
| Missing | 18 | 13 | 3 | 3 | ||||||
| CD 45 | ||||||||||
| Low | 113 | 80 | 21 | 33 | 0.745 | 87 | 81 | NR | 61 | 0.530 |
| High | 19 | 13 | 25 | 37 | 18 | 17 | NR | 67 | ||
| Missing | 9 | 6 | 3 | 3 | ||||||
Abbreviations: NR, not reached.
Figure 3. Disease-specific survival curves for CD3+, CD4+, CD8+, CD20+ and CD45+ lymphocytes in STS with wide resection margins.
Improved survival was seen in patients younger than 60 years (P = 0.005), in tumors of histological grade 1 and 2 (P = 0.011), in tumors less than 5 cm (P = 0.018) and in patients who received chemotherapy (P = 0.024). This was shown through subgroup analysis of patients with high CD20+ lymphocytes in tumor and wide resection margins, Table 3. The same statistical trend was seen for gender, nationality, histology, tumor localization and in patients with or without radiotherapy, but these were not statistically significant (data not shown). There were no significant differences in the expression of the different immunomarkers in the different tumor groups (data not shown).
Table 3. Results of subgroup analysis of patients with CD20+ lymphocytes in tumor and wide resection margins, n = 108.
| Subgroup | Patients(n) | Patients(%) | Median survival(months) | 5-Year survival(%) | P |
| Age | |||||
| <60, CD20 Low | 49 | 79 | - | 56 | 0.005 |
| <60, CD20 High | 11 | 18 | -* | 100 | |
| Missing | 2 | 3 | |||
| >60, CD20 Low | 34 | 74 | NR | 54 | 0.347 |
| >60, CD20 High | 11 | 24 | NR | 73 | |
| Missing | 1 | 2 | |||
| Histological grade | |||||
| 1 or 2, CD20 Low | 52 | 76 | - | 67 | 0.011 |
| 1 or 2, CD20 High | 14 | 21 | - | 100 | |
| Missing | 2 | 3 | |||
| 3, CD20 Low | 31 | 78 | 28 | 34 | 0.155 |
| 3, CD20 High | 8 | 20 | NR | 63 | |
| Missing | 1 | 3 | |||
| Tumor size | |||||
| <5 cm, CD20 Low | 28 | 70 | - | 63 | 0.018 |
| <5 cm, CD20 High | 10 | 25 | - | 100 | |
| Missing | 2 | 5 | |||
| >5 cm, CD20 Low | 53 | 78 | 63 | 52 | 0.169 |
| >5 cm, CD20 High | 12 | 18 | NR | 75 | |
| Missing | 3 | 4 | |||
| Chemotherapy | |||||
| Yes, CD20 Low | 24 | 80 | - | 82 | 0.024 |
| Yes, CD20 High | 5 | 17 | - | 100 | |
| Missing | 1 | 3 | |||
| No, CD20 Low | 59 | 76 | NR | 59 | 0.080 |
| No, CD20 High | 17 | 22 | NR | 46 | |
| Missing | 2 | 3 |
* Median survival is not computed because all cases are censored.
Abbreviations: NR, not reached.
Multivariate analyses
Significant demographic, clinicopathological, and lymphocyte infiltrate variables from the univariate analyses were entered into the multivariate Cox regression analysis. An independent positive prognostic factor for improved DSS in patients with wide resection margin was a high number of CD20+ lymphocytes in the tumor (HR 5.5, CI 95% 1.62–18.61, P = 0.006).
Independent negative prognostic variables were Russian nationality (P = 0.020), high malignancy grade (P = 0.016) and metastasis at time of diagnosis (P = 0.001, Table 4). In patients with non-wide resection margins (n = 141) increasing numbers of CD3+ lymphocytes was an independent negative prognostic factor for DSS, (HR 2.2, CI 95% 1.25–3.89, P = 0.007), (Table 4).
Table 4. Results of Cox regression analysis summarizing some significant independent prognostic factors in patients with soft tissue sarcomas, N = 249.
| Non-wide resections margins, n = 141 | Wide resection margins, n = 108 | |||||
| Factor | Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P |
| Nationality | ||||||
| Norwegian | 1.000 | 1.000 | ||||
| Russian | 1.635 | 0.978–2.731 | 0.061 | 2.246 | 1.135–4.444 | 0.020 |
| Tumor size | 0.428* | 0.874* | ||||
| ≤5 cm | 1.000 | 1.000 | ||||
| 5–10 cm | 0.826 | 0.463–1.605 | 1.217 | 0.547–2.708 | ||
| >10 cm | 1.232 | 0.690–2.199 | 1.045 | 0.389–2.806 | ||
| Malignancy grade FNCLCC | 0.024* | 0.016* | ||||
| 1 | 1.000 | 1.000 | ||||
| 2 | 1.237 | 0.611–2.506 | 3.464 | 1.081–11.096 | 0.036 | |
| 3 | 2.214 | 1.108–4.425 | 5.046 | 1.656–15.376 | 0.004 | |
| Metastasis at time of diagnosis | ||||||
| No | 1.000 | 1.000 | ||||
| Yes | 3.651 | 2.081–6.409 | <0.001 | 3.872 | 1.696–8.836 | 0.001 |
| CD3 | ||||||
| Low | 1.000 | NIA | ||||
| High | 2.202 | 1.245–3.893 | 0.007 | |||
| CD4 | ||||||
| Low | NIA | 4.126 | 0.551–30.895 | 0.168 | ||
| High | 1.000 | |||||
| CD20 | ||||||
| Low | NIA | 5.503 | 1.627–18.606 | 0.006 | ||
| High | 1.000 | |||||
* Overall significance as a prognostic factor.
Abbreviations: NIA, not included in analysis.
Discussion
In this large scale study, we evaluated whether there is an association between the prevalence of CD3+, CD4+, CD8+, CD20+ and CD45+ lymphocytes in tumors and survival prognosis in 249 non-GIST STS patients. Interestingly, high intensities of CD20+ cells in tumors were an independent positive prognostic factor in patients with wide resection margins.
To our knowledge, this is the first report on CD20 expression in non-GIST STS and the first evidence of its possible clinical relevance in non-GIST STS patients with wide resection margins. This may suggest that CD20+ cells in the tumor are mediating a strong anti-tumor immune response in STS, but this effect is not strong enough to improve survival in patients without wide resection margins.
Activation of the adaptive immune system may suppress malignant cells, whereas activation of various types of innate immune cells may promote tumor growth[14]. The adaptive immunity, orchestrated by antigen-specific T and B-lymphocytes, inhibits tumor growth through both direct killing by cytotoxic T-lymphocytes, and a combination of cytokine and antibody mediated tumor cell lysis[14]. Cancer infiltration by tumor reactive T-lymphocytes is required for efficient tumor eradication[15]. However, cancer cells can escape the immune system in several ways including suppression of cytotoxic T-cells, by regulatory T-cells and by accumulation of myeloid suppressor cells[15]–[17].
Tumor-infiltration CD3+ cells are reported to be strongly associated with favorable prognosis in epithelial tumors in several studies[18]–[22]. The CD3+ cell is an independent positive predictor of response to neoadjuvant chemotherapy in breast cancer[23]. Low numbers of CD3+ lymphocytes predicted shorter disease-free survival in colon cancer[24] and cervical cancers[25]. However, T-cell parameters including CD3 values showed no correlation with survival in cases of metastatic ovarian carcinoma[26]. Accordingly, we did not find any such association in our mesenchymal material in patients with wide resection margins, but CD3 was a negative prognostic factor in patients with non-wide resection margins.
The role of CD4+ T and B lymphocytes is controversial in many cancers including STS; CD4+ cells in the absence of the CD8+ cytotoxic T cells are critical and sufficient for NKT cell-dependent rejection of experimental tumours[27]. In lung cancer the prognostic impact of CD4 is controversial[28], [29], but in our material CD4+ cells were a positive prognostic factor in univariate analyses.
CD8+ cells in malignant tumors have been associated with a better survival in many types of cancer including: non-small cell lung carcinoma; carcinomas of the endometrium, bile duct, colon, oesophagus, urothelium; and uveal melanoma and follicular lymphoma[28], [30]–[37]. However, the role of CD8+ lymphocytes in STS is controversial and most of the studies contain relatively few cases. There was a positive correlation between a high density of CD4+ and CD8+ lymphocytes in stroma and improved disease-specific survival in non-small cell lung cancer[28]. In our material CD8 was not a statistically significant factor (P = 0.15).
CD20+ cells are associated with a better survival in lung cancer, cervical cancer, prostate cancer and ovarian cancer[25], [28], [38]–[40]. CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma[41]. On the other hand, B-cell infiltration detected by flowcytometry with CD19 were correlated with unfavourable outcome in metastatic ovarian carcinoma[26]. In our material high density of CD20+ lymphocytes was an independent positive prognostic indicator.
In cervical cancer no significant impact of CD45+ cells were seen [25], neither was it in non-GIST STS in this study.
The optimal chance for curing localized STS is based on wide resection surgery. Given that of the majority of STS patients succumb to this disease within 5 years, there is an apparent need for better systemic therapy including novel molecularly targeted therapies[42]. In our study, there was a 33% 5-year survival in the group with non-wide resection margins and 62% of those with wide resection margins.
Among STS patients who have had wide resection margins, it will be essential to identify those who will relapse and succumb this disease as these patients may benefit from adjuvant therapy, including immunotherapy. Until now adjuvant chemotherapy has been controversial due to inadequate selection criteria.
The human immune system contains specialized cells that are able to eliminate cancer cells[26], and tumor-infiltrating B-cells are able to produce tumor-specific antibodies[43]. Through external stimulation of the immune response, these cells may have the potential to aid the immune system in destroying single tumor cells and micro-metastases after surgery. This topic is investigated in the ongoing international osteosarcoma protocol EURAMOS where those who respond well to chemotherapy are randomized to receive interferon or no interferon, in an attempt to improve the immune response (http://www.ctu.mrc.ac.uk/euramos/).
In conclusion, high density of CD20+ lymphocytes in STS with wide resection margins is an independent positive prognostic indicator for these patients. Further research to define if CD20+ cells can modify tumors in a way that reduces disease progression and metastatic potential is needed.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The work was supported by the Northern Norway Regional Health Authority (Helse Nord RHF, http://www.helse-nord.no/?lang=en_US). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gustafson P. Soft tissue sarcoma. Epidemiology and prognosis in 508 patients. Acta Orthop Scand Suppl. 1994;259:1–31. [PubMed] [Google Scholar]
- 2.Engellau J, Anderson H, Rydholm A, Bauer HC, Hall KS, et al. Time dependence of prognostic factors for patients with soft tissue sarcoma: a Scandinavian Sarcoma Group Study of 338 malignant fibrous histiocytomas. Cancer. 2004;100:2233–2239. doi: 10.1002/cncr.20254. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson IC, Whitwell DJ, Battistuta D, Thompson B, Strobel N, et al. Surgical margin and its influence on survival in soft tissue sarcoma. ANZ J Surg. 2006;76:104–109. doi: 10.1111/j.1445-2197.2006.03615.x. [DOI] [PubMed] [Google Scholar]
- 4.Kiatisevi P, Asavamongkolkul A, Phimolsarnti R, Waikakul S, Benjarassamerote S. The outcomes and prognostic factors of patients with soft-tissue sarcoma. J Med Assoc Thai. 2006;89:334–342. [PubMed] [Google Scholar]
- 5.Koea JB, Leung D, Lewis JJ, Brennan MF. Histopathologic type: an independent prognostic factor in primary soft tissue sarcoma of the extremity? Ann Surg Oncol. 2003;10:432–440. doi: 10.1245/aso.2003.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Mendenhall WM, Zlotecki RA, Hochwald SN, Hemming AW, Grobmyer SR, et al. Retroperitoneal soft tissue sarcoma. Cancer. 2005;104:669–675. doi: 10.1002/cncr.21264. [DOI] [PubMed] [Google Scholar]
- 7.Raney RB, Jr, Crist WM, Maurer HM, Foulkes MA. Prognosis of children with soft tissue sarcoma who relapse after achieving a complete response. A report from the Intergroup Rhabdomyosarcoma Study I. Cancer. 1983;52:44–50. doi: 10.1002/1097-0142(19830701)52:1<44::aid-cncr2820520110>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Yang RS, Lane JM, Eilber FR, Dorey FJ, al Shaikh R, et al. High grade soft tissue sarcoma of the flexor fossae. Size rather than compartmental status determine prognosis. Cancer. 1995;76:1398–1405. doi: 10.1002/1097-0142(19951015)76:8<1398::aid-cncr2820760815>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, et al. Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:739–747. doi: 10.1016/s0360-3016(03)00714-4. [DOI] [PubMed] [Google Scholar]
- 10.Ottaiano A, De Chiara A, Fazioli F, Talamanca AA, Mori S, et al. Biological prognostic factors in adult soft tissue sarcomas. Anticancer Res. 2005;25:4519–4526. [PubMed] [Google Scholar]
- 11.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 12.Nocito A, Kononen J, Kallioniemi OP, Sauter G. Tissue microarrays (TMAs) for high-throughput molecular pathology research. Int J Cancer. 2001;94:1–5. doi: 10.1002/ijc.1385. [DOI] [PubMed] [Google Scholar]
- 13.Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 2001;10:657–662. doi: 10.1093/hmg/10.7.657. [DOI] [PubMed] [Google Scholar]
- 14.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 15.Mukai S, Kjaergaard J, Shu S, Plautz GE. Infiltration of tumors by systemically transferred tumor-reactive T lymphocytes is required for antitumor efficacy. Cancer Res. 1999;59:5245–5249. [PubMed] [Google Scholar]
- 16.Costello RT, Gastaut JA, Olive D. Tumor escape from immune surveillance. Arch Immunol Ther Exp (Warsz) 1999;47:83–88. [PubMed] [Google Scholar]
- 17.Enarsson K, Lundin BS, Johnsson E, Brezicka T, Quiding-Jarbrink M. CD4+ CD25high regulatory T cells reduce T cell transendothelial migration in cancer patients. Eur J Immunol. 2007;37:282–291. doi: 10.1002/eji.200636183. [DOI] [PubMed] [Google Scholar]
- 18.Gimotty PA, Zhang L, Alagkiozidis I, Cadungog M, Adams S, et al. Immune prognostic factors in ovarian cancer: lessons from translational research. Dis Markers. 2007;23:445–452. doi: 10.1155/2007/508350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev. 2008;222:101–116. doi: 10.1111/j.1600-065X.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 20.Raspollini MR, Castiglione F, Rossi DD, Amunni G, Villanucci A, et al. Tumour-infiltrating gamma/delta T-lymphocytes are correlated with a brief disease-free interval in advanced ovarian serous carcinoma. Ann Oncol. 2005;16:590–596. doi: 10.1093/annonc/mdi112. [DOI] [PubMed] [Google Scholar]
- 21.Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108:415–420. doi: 10.1016/j.ygyno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 23.Denkert C, Loibl S, Noske A, Roller M, Muller BM, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 24.Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, et al. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270–1279. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ancuta E, Ancuta C, Zugun-Eloae F, Iordache C, Chirieac R, et al. Predictive value of cellular immune response in cervical cancer. Rom J Morphol Embryol. 2009;50:651–655. [PubMed] [Google Scholar]
- 26.Dong HP, Elstrand MB, Holth A, Silins I, Berner A, et al. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol. 2006;125:451–458. [PubMed] [Google Scholar]
- 27.Hong C, Lee H, Oh M, Kang CY, Hong S, et al. CD4+ T cells in the absence of the CD8+ cytotoxic T cells are critical and sufficient for NKT cell-dependent tumor rejection. J Immunol. 2006;177:6747–6757. doi: 10.4049/jimmunol.177.10.6747. [DOI] [PubMed] [Google Scholar]
- 28.Al Shibli KI, Donnem T, Al Saad S, Persson M, Bremnes RM, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 29.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eerola AK, Soini Y, Paakko P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin Cancer Res. 2000;6:1875–1881. [PubMed] [Google Scholar]
- 31.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 32.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 33.Oshikiri T, Miyamoto M, Shichinohe T, Suzuoki M, Hiraoka K, et al. Prognostic value of intratumoral CD8+ T lymphocyte in extrahepatic bile duct carcinoma as essential immune response. J Surg Oncol. 2003;84:224–228. doi: 10.1002/jso.10321. [DOI] [PubMed] [Google Scholar]
- 34.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 35.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staibano S, Mascolo M, Tranfa F, Salvatore G, Mignogna C, et al. Tumor infiltrating lymphocytes in uveal melanoma: a link with clinical behavior? Int J Immunopathol Pharmacol. 2006;19:171–179. [PubMed] [Google Scholar]
- 37.Wahlin BE, Sander B, Christensson B, Kimby E. CD8+ T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res. 2007;13:388–397. doi: 10.1158/1078-0432.CCR-06-1734. [DOI] [PubMed] [Google Scholar]
- 38.Karja V, Aaltomaa S, Lipponen P, Isotalo T, Talja M, et al. Tumour-infiltrating lymphocytes: A prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res. 2005;25:4435–4438. [PubMed] [Google Scholar]
- 39.Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelletier MP, Edwardes MD, Michel RP, Halwani F, Morin JE. Prognostic markers in resectable non-small cell lung cancer: a multivariate analysis. Can J Surg. 2001;44:180–188. [PMC free article] [PubMed] [Google Scholar]
- 41.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, et al. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thornton K. Chemotherapeutic management of soft tissue sarcoma. Surg Clin North Am. 2008;88:647–60, viii. doi: 10.1016/j.suc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Sikora K, Alderson T, Ellis J, Phillips J, Watson J. Human hybridomas from patients with malignant disease. Br J Cancer. 1983;47:135–145. doi: 10.1038/bjc.1983.16. [DOI] [PMC free article] [PubMed] [Google Scholar]