Abstract
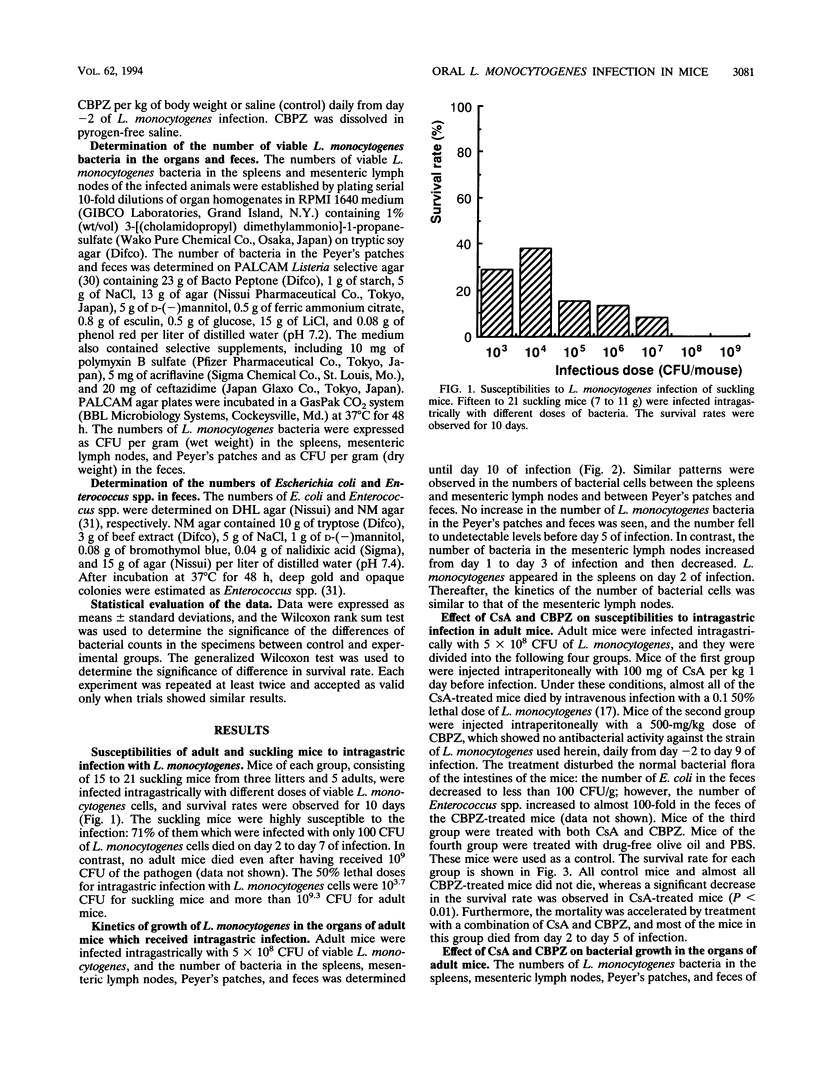
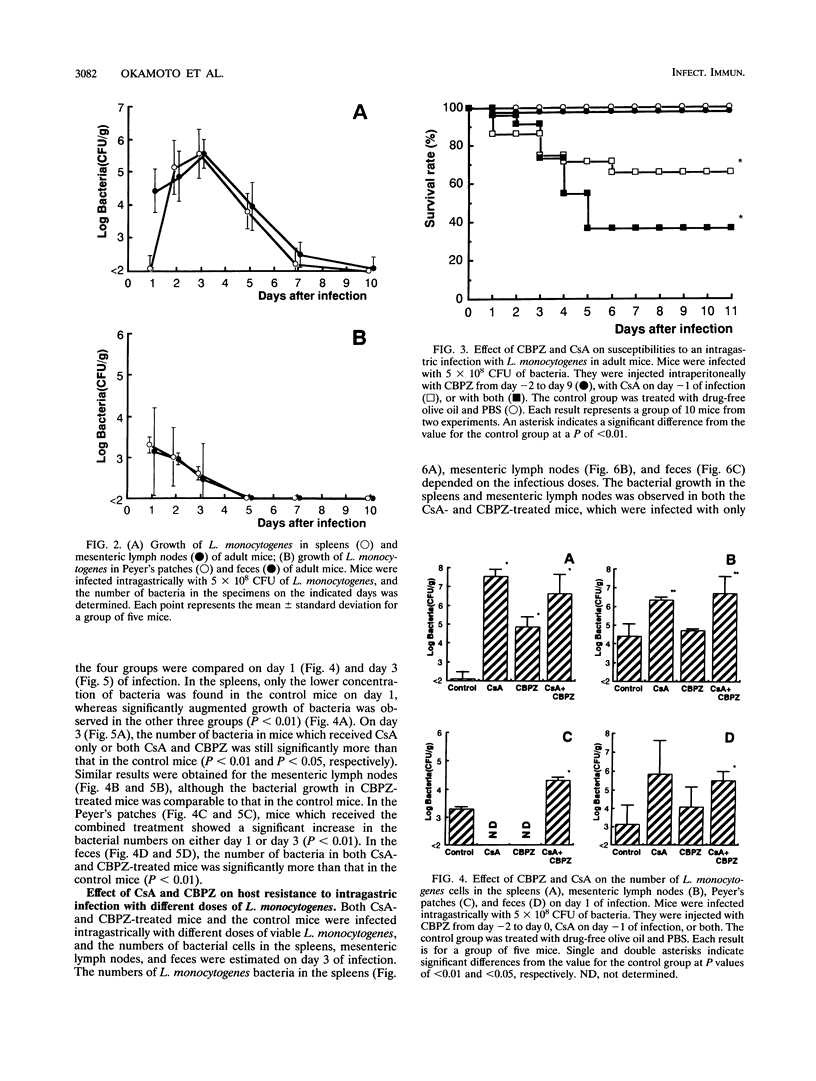
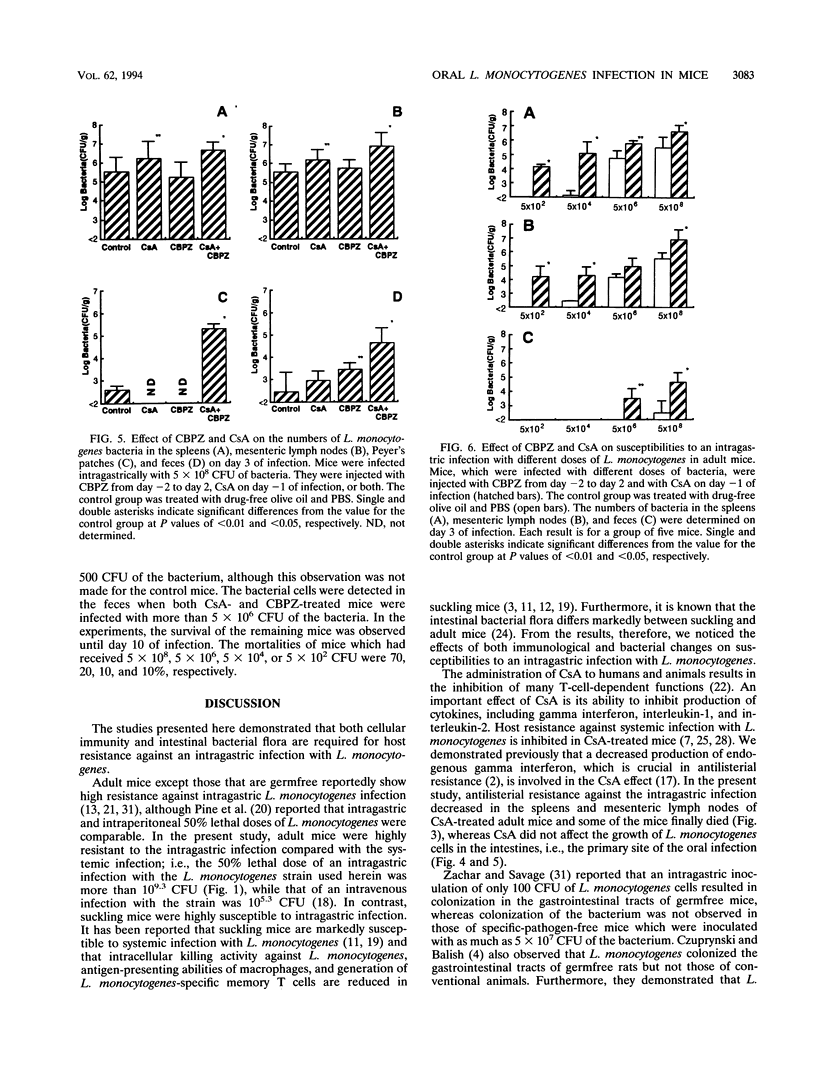
Suckling and adult mice were infected intragastrically with different doses of viable Listeria monocytogenes. The 50% lethal dose for the intragastric infection was 10(3.7) CFU for suckling mice, while adult mice were highly resistant and the 50% lethal dose was more than 10(9.3) CFU. When adult mice were infected intragastrically with 5 x 10(8) CFU of L. monocytogenes, no mice died. However, 35% of adult mice died when they were treated with cyclosporin A 1 day before infection. Although mice did not die when treated with an L. monocytogenes-resistant broad-spectrum cephalosporin, sodium cefbuperazone, before and during infection, the number of L. monocytogenes bacteria increased in the feces. The sodium cefbuperazone treatment of mice resulted in superinfection, i.e., a marked decrease of Escherichia coli and an increase of Enterococcus spp. in the intestines. Furthermore, host resistance against the intragastric infection markedly decreased when the mice were treated with both drugs. The growth of L. monocytogenes was augmented in the spleens, mesenteric lymph nodes, Peyer's patches, and feces, and the mortality of the mice was 65%. These results suggest that both cellular immunity and the intestinal bacterial flora are required for host resistance against oral L. monocytogenes infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Nakane A., Minagawa T. Recombinant murine gamma interferon induces enhanced resistance to Listeria monocytogenes infection in neonatal mice. Infect Immun. 1989 Aug;57(8):2345–2349. doi: 10.1128/iai.57.8.2345-2349.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Balish E. Pathogenesis of Listeria monocytogenes for gnotobiotic rats. Infect Immun. 1981 Apr;32(1):323–331. doi: 10.1128/iai.32.1.323-331.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Hügin A. W., Cerny A., Wrann M., Hengartner H., Zinkernagel R. M. Effect of cyclosporin A on immunity to Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):12–17. doi: 10.1128/iai.52.1.12-17.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampelmacher E. H., Huysinga W. T., van Noorle Jansen L. M. The presence of Listeria monocytogenes in feces of pregnant women and neonates. Zentralbl Bakteriol Orig A. 1972 Nov;222(2):258–262. [PubMed] [Google Scholar]
- Kampelmacher E. H., van Noorle Jansen L. M. Isolation of Listeria monocytogenes from faeces of clinically healthy humans and animals. Zentralbl Bakteriol Orig. 1969;211(3):353–359. [PubMed] [Google Scholar]
- Linnan M. J., Mascola L., Lou X. D., Goulet V., May S., Salminen C., Hird D. W., Yonekura M. L., Hayes P., Weaver R. Epidemic listeriosis associated with Mexican-style cheese. N Engl J Med. 1988 Sep 29;319(13):823–828. doi: 10.1056/NEJM198809293191303. [DOI] [PubMed] [Google Scholar]
- Lu C. Y., Calamai E. G., Unanue E. R. A defect in the antigen-presenting function of macrophages from neonatal mice. Nature. 1979 Nov 15;282(5736):327–329. doi: 10.1038/282327a0. [DOI] [PubMed] [Google Scholar]
- Lu C. Y., Unanue E. R. Ontogeny of murine macrophages: functions related to antigen presentation. Infect Immun. 1982 Apr;36(1):169–175. doi: 10.1128/iai.36.1.169-175.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Carter P. B. Cell-mediated immunity to intestinal infection. Infect Immun. 1980 May;28(2):516–523. doi: 10.1128/iai.28.2.516-523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Ehlers S., Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988 Aug;56(8):1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Medoff G., Leech I., Wennersten C., Kunz L. J. Antibiotic synergism against Listeria monocytogenes. Antimicrob Agents Chemother. 1972 Jan;1(1):30–34. doi: 10.1128/aac.1.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Yasuda I. Induction of alpha/beta interferon and gamma interferon in mice infected with Listeria monocytogenes during pregnancy. Infect Immun. 1985 Dec;50(3):877–880. doi: 10.1128/iai.50.3.877-880.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Yasuda I., Yu C., Kato K. Prevention by gamma interferon of fatal infection with Listeria monocytogenes in mice treated with cyclosporin A. Infect Immun. 1988 Aug;56(8):2011–2015. doi: 10.1128/iai.56.8.2011-2015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Okamoto M., Asano M., Kohanawa M., Minagawa T. An anti-CD3 monoclonal antibody protects mice against a lethal infection with Listeria monocytogenes through induction of endogenous cytokines. Infect Immun. 1993 Jul;61(7):2786–2792. doi: 10.1128/iai.61.7.2786-2792.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara R., Mitsuyama M., Miyata M., Nomoto K. Ontogeny of macrophage-mediated protection against Listeria monocytogenes. Infect Immun. 1985 Jun;48(3):763–768. doi: 10.1128/iai.48.3.763-768.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., Malcolm G. B., Plikaytis B. D. Listeria monocytogenes intragastric and intraperitoneal approximate 50% lethal doses for mice are comparable, but death occurs earlier by intragastric feeding. Infect Immun. 1990 Sep;58(9):2940–2945. doi: 10.1128/iai.58.9.2940-2945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll J. T., Czuprynski C. J. Hemolysin is required for extraintestinal dissemination of Listeria monocytogenes in intragastrically inoculated mice. Infect Immun. 1990 Sep;58(9):3147–3150. doi: 10.1128/iai.58.9.3147-3150.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel B. Pharmacology of cyclosporine. VI. Cellular activation: regulation of intracellular events by cyclosporine. Pharmacol Rev. 1990 Sep;41(3):407–422. [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Davis C. E. Models of T cell deficiency in listeriosis: the effects of cortisone and cyclosporin A on normal and nude BALB/c mice. J Immunol. 1983 Jul;131(1):450–453. [PubMed] [Google Scholar]
- Schuchat A., Deaver K., Hayes P. S., Graves L., Mascola L., Wenger J. D. Gastrointestinal carriage of Listeria monocytogenes in household contacts of patients with listeriosis. J Infect Dis. 1993 May;167(5):1261–1262. doi: 10.1093/infdis/167.5.1261. [DOI] [PubMed] [Google Scholar]
- Strauss R., Heymer B., Hof H. Effects of cyclosporin A on experimental infection with Listeria monocytogenes. Clin Exp Immunol. 1985 Dec;62(3):491–498. [PMC free article] [PubMed] [Google Scholar]
- Traub W. H. Perinatal listeriosis. Tolerance of a clinical isolate of Listeria monocytogenes for ampicillin and resistance against cefotaxime. Chemotherapy. 1981;27(6):423–431. doi: 10.1159/000238012. [DOI] [PubMed] [Google Scholar]
- Zachar Z., Savage D. C. Microbial interference and colonization of the murine gastrointestinal tract by Listeria monocytogenes. Infect Immun. 1979 Jan;23(1):168–174. doi: 10.1128/iai.23.1.168-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Netten P., Perales I., van de Moosdijk A., Curtis G. D., Mossel D. A. Liquid and solid selective differential media for the detection and enumeration of L. monocytogenes and other Listeria spp. Int J Food Microbiol. 1989 Jul;8(4):299–316. doi: 10.1016/0168-1605(89)90001-9. [DOI] [PubMed] [Google Scholar]


