Abstract
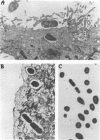
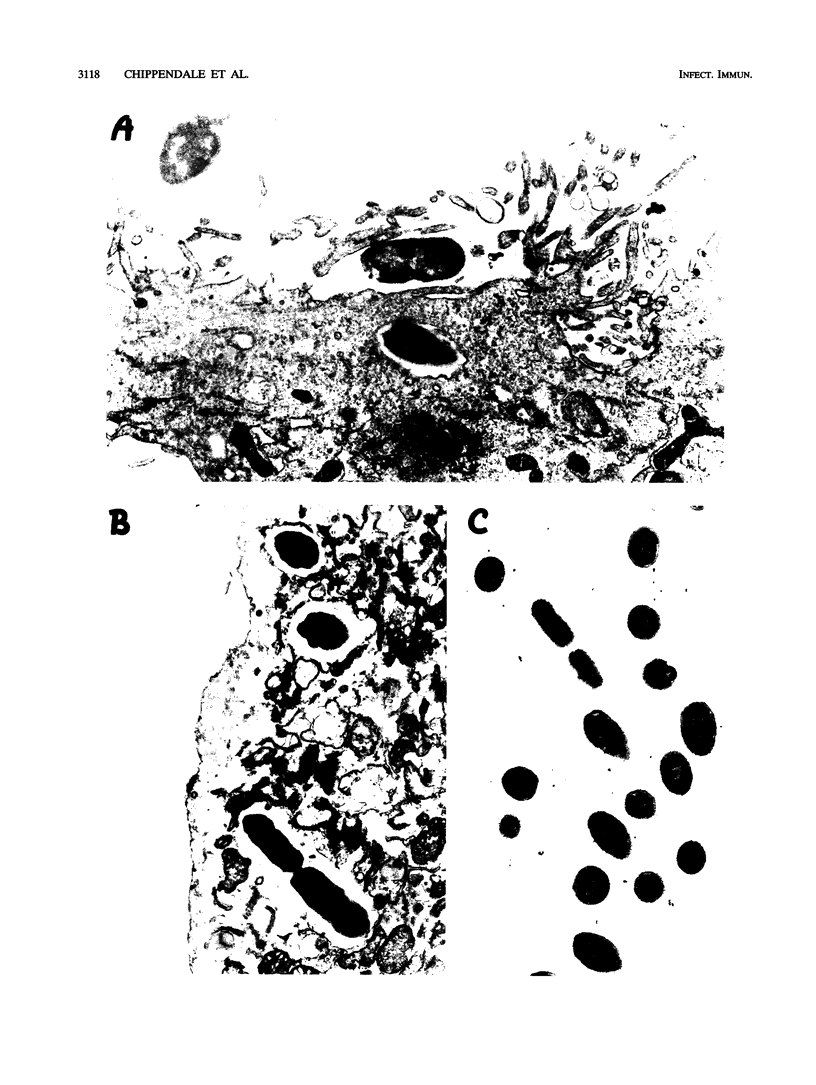
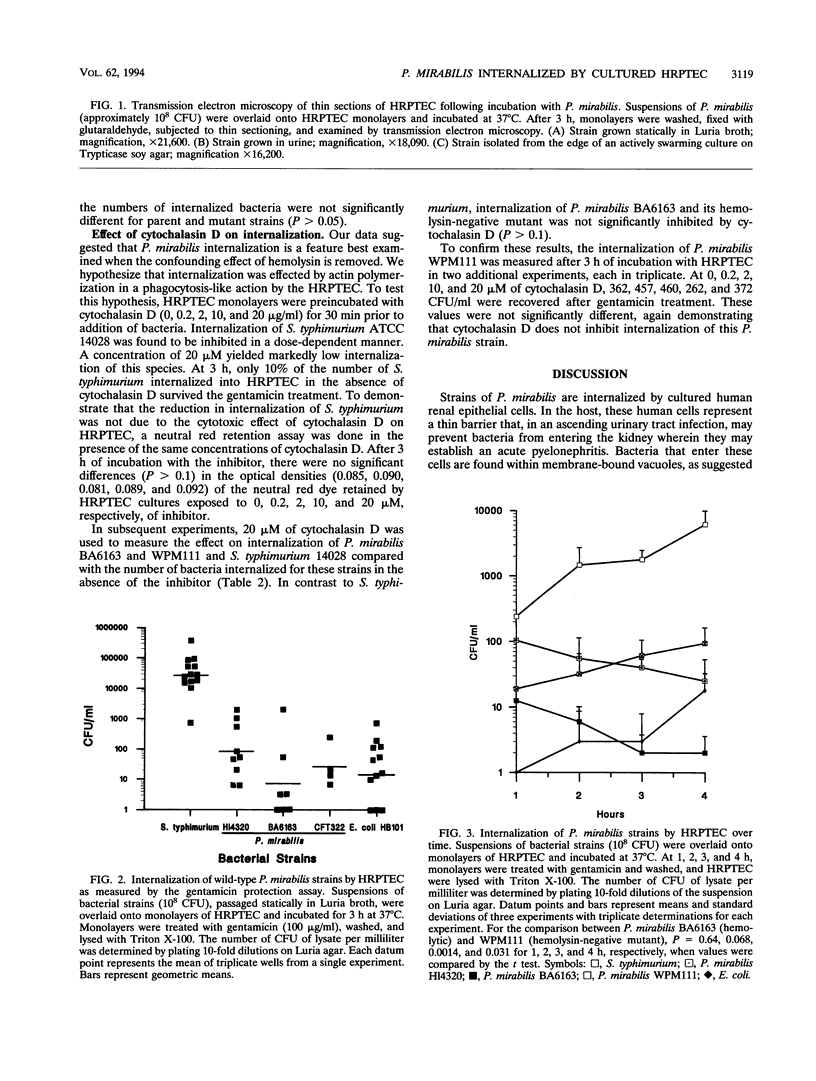
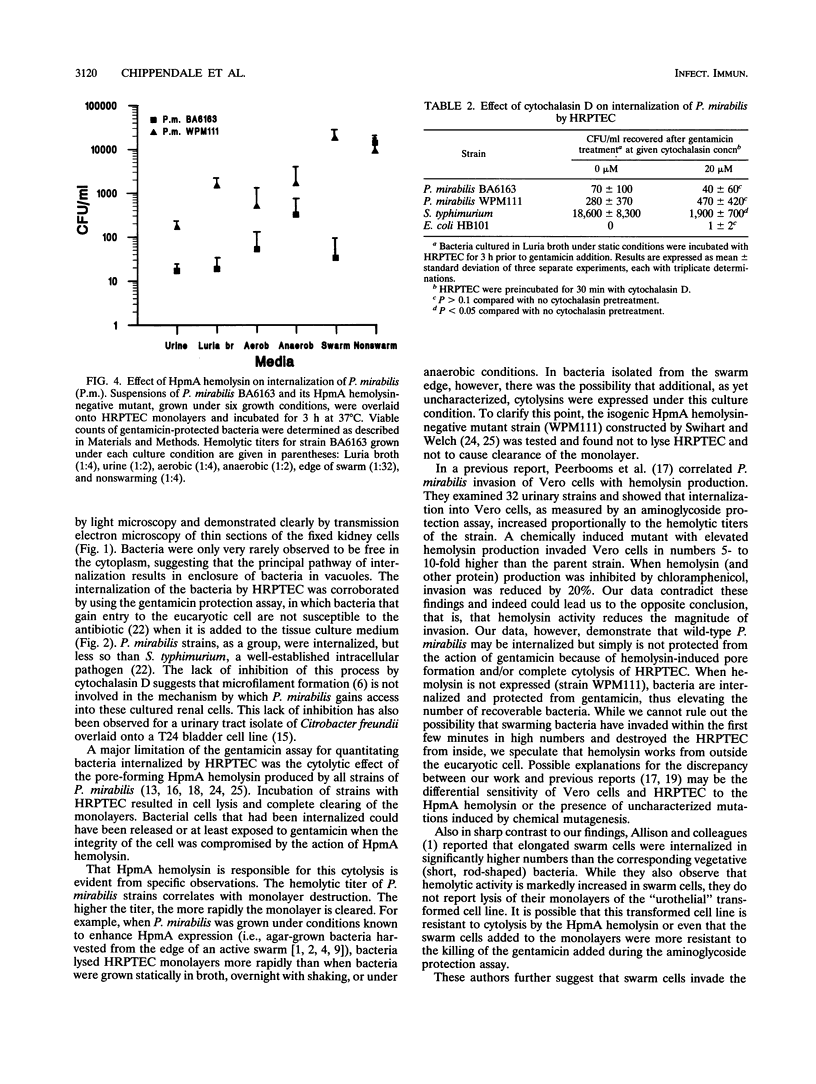
Proteus mirabilis, a common agent of bacteriuria in humans, causes acute pyelonephritis and bacteremia. Renal epithelium provides a barrier between luminal organisms and the renal interstitium. We have hypothesized that P. mirabilis may be internalized into renal epithelium. To test this hypothesis, we added suspensions of three P. mirabilis strains (10(8) CFU) to confluent monolayers of primary cultures of human renal proximal tubular epithelial cells (HRPTEC) and, after 3 h, found the bacteria internalized within membrane-bound vacuoles by light and electron microscopy. Internalization of bacteria by HRPTEC was corroborated by using the gentamicin protection assay. Cytolysis of HRPTEC by the HpmA hemolysin, however, was a confounding factor in this assay, and therefore a hemolysin-negative hpmA mutant was used in subsequent experiments. The nonhemolytic mutant WPM111 did not disrupt the monolayer and was recovered in numbers that were 10- to 100-fold higher than those of the hemolytic parent BA6163. Cytochalasin D (20 micrograms/ml) inhibited internalization of Salmonella typhimurium but not that of P. mirabilis, suggesting that the latter species enters HRPTEC by a mechanism that is not dependent on actin polymerization. We suggest that HpmA hemolysin-mediated cytotoxicity and internalization of bacteria by HRPTEC may play a role in the development of Proteus pyelonephritis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison C., Coleman N., Jones P. L., Hughes C. Ability of Proteus mirabilis to invade human urothelial cells is coupled to motility and swarming differentiation. Infect Immun. 1992 Nov;60(11):4740–4746. doi: 10.1128/iai.60.11.4740-4746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison C., Lai H. C., Hughes C. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol Microbiol. 1992 Jun;6(12):1583–1591. doi: 10.1111/j.1365-2958.1992.tb00883.x. [DOI] [PubMed] [Google Scholar]
- BRAUDE A. I., SIEMIENSKI J. Role of bacterial urease in experimental pyelonephritis. J Bacteriol. 1960 Aug;80:171–179. doi: 10.1128/jb.80.2.171-179.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich H., Borenfreund E. Cytotoxicity of T-2 toxin and its metabolites determined with the neutral red cell viability assay. Appl Environ Microbiol. 1991 Jul;57(7):2101–2103. doi: 10.1128/aem.57.7.2101-2103.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R., Erskine D., Flaherty D. Proteus mirabilis mutants defective in swarmer cell differentiation and multicellular behavior. J Bacteriol. 1991 Oct;173(19):6279–6288. doi: 10.1128/jb.173.19.6279-6288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Newman B., Utsalo S. J., Trifillis A. L., Hebel J. R., Warren J. W. Internalization of Escherichia coli into human kidney epithelial cells: comparison of fecal and pyelonephritis-associated strains. J Infect Dis. 1994 Apr;169(4):831–838. doi: 10.1093/infdis/169.4.831. [DOI] [PubMed] [Google Scholar]
- Fairley K. F., Bond A. G., Brown R. B., Habersberger P. Simple test to determine the site of urinary-tract infection. Lancet. 1967 Aug 26;2(7513):427–428. doi: 10.1016/s0140-6736(67)90849-5. [DOI] [PubMed] [Google Scholar]
- Falkinham J. O., 3rd, Hoffman P. S. Unique developmental characteristics of the swarm and short cells of Proteus vulgaris and Proteus mirabilis. J Bacteriol. 1984 Jun;158(3):1037–1040. doi: 10.1128/jb.158.3.1037-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R. Hemagglutinin, urease, and hemolysin production by Proteus mirabilis from clinical sources. J Infect Dis. 1990 Mar;161(3):525–530. doi: 10.1093/infdis/161.3.525. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R., Swihart K. G., Welch R. A. Cytotoxicity of the HpmA hemolysin and urease of Proteus mirabilis and Proteus vulgaris against cultured human renal proximal tubular epithelial cells. Infect Immun. 1991 Jun;59(6):2036–2042. doi: 10.1128/iai.59.6.2036-2042.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Green D. M., Trifillis A. L., Johnson D. E., Chippendale G. R., Lockatell C. V., Jones B. D., Warren J. W. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990 May;58(5):1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelschlaeger T. A., Guerry P., Kopecko D. J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Investigation of the haemolytic activity of Proteus mirabilis strains. Antonie Van Leeuwenhoek. 1983 Apr;49(1):1–11. doi: 10.1007/BF00457874. [DOI] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Uropathogenic properties of Proteus mirabilis and Proteus vulgaris. J Med Microbiol. 1985 Feb;19(1):55–60. doi: 10.1099/00222615-19-1-55. [DOI] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Vero cell invasiveness of Proteus mirabilis. Infect Immun. 1984 Mar;43(3):1068–1071. doi: 10.1128/iai.43.3.1068-1071.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozalski A., Kotełko K. Hemolytic activity and invasiveness in strains of Proteus penneri. J Clin Microbiol. 1987 Jun;25(6):1094–1096. doi: 10.1128/jcm.25.6.1094-1096.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setia U., Serventi I., Lorenz P. Bacteremia in a long-term care facility. Spectrum and mortality. Arch Intern Med. 1984 Aug;144(8):1633–1635. [PubMed] [Google Scholar]
- Small P. L., Isberg R. R., Falkow S. Comparison of the ability of enteroinvasive Escherichia coli, Salmonella typhimurium, Yersinia pseudotuberculosis, and Yersinia enterocolitica to enter and replicate within HEp-2 cells. Infect Immun. 1987 Jul;55(7):1674–1679. doi: 10.1128/iai.55.7.1674-1679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets F., Gower P. E. The site of infection in 133 patients with bacteriuria. Clin Nephrol. 1973 Sep-Oct;1(5):290–296. [PubMed] [Google Scholar]
- Swihart K. G., Welch R. A. Cytotoxic activity of the Proteus hemolysin HpmA. Infect Immun. 1990 Jun;58(6):1861–1869. doi: 10.1128/iai.58.6.1861-1869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swihart K. G., Welch R. A. The HpmA hemolysin is more common than HlyA among Proteus isolates. Infect Immun. 1990 Jun;58(6):1853–1860. doi: 10.1128/iai.58.6.1853-1860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifillis A. L., Regec A. L., Trump B. F. Isolation, culture and characterization of human renal tubular cells. J Urol. 1985 Feb;133(2):324–329. doi: 10.1016/s0022-5347(17)48932-4. [DOI] [PubMed] [Google Scholar]
- Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982 Dec;146(6):719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]