Abstract
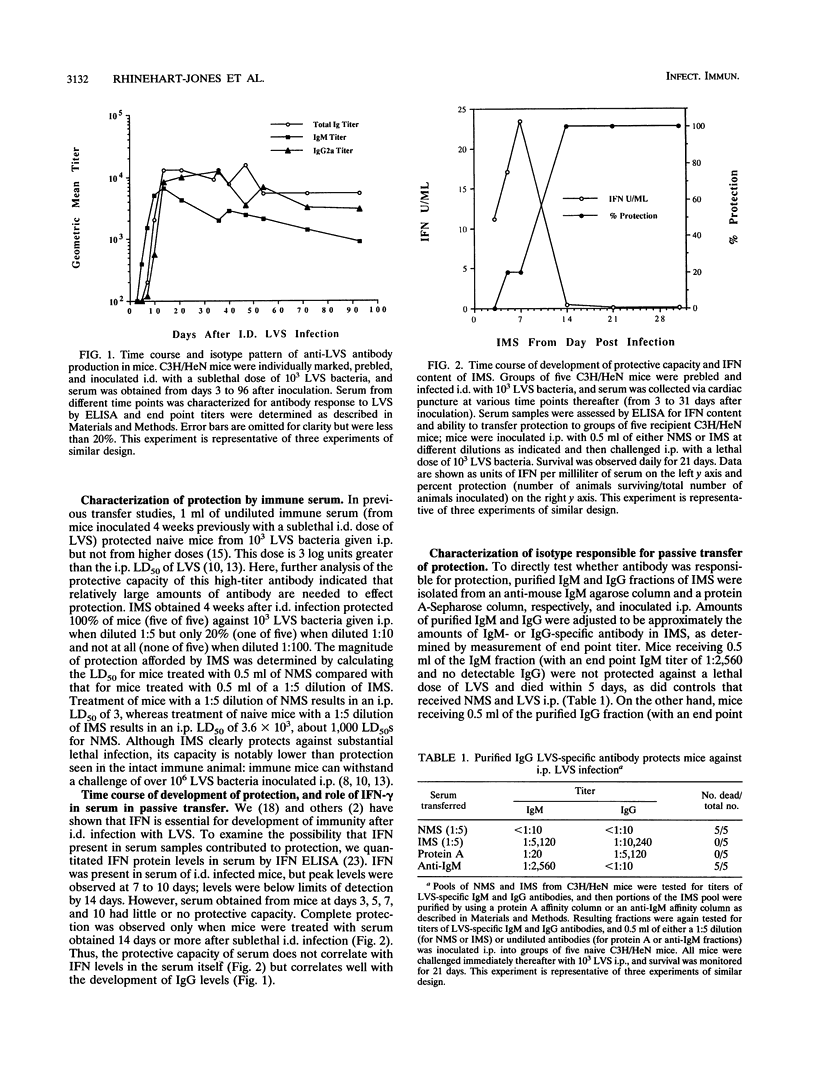
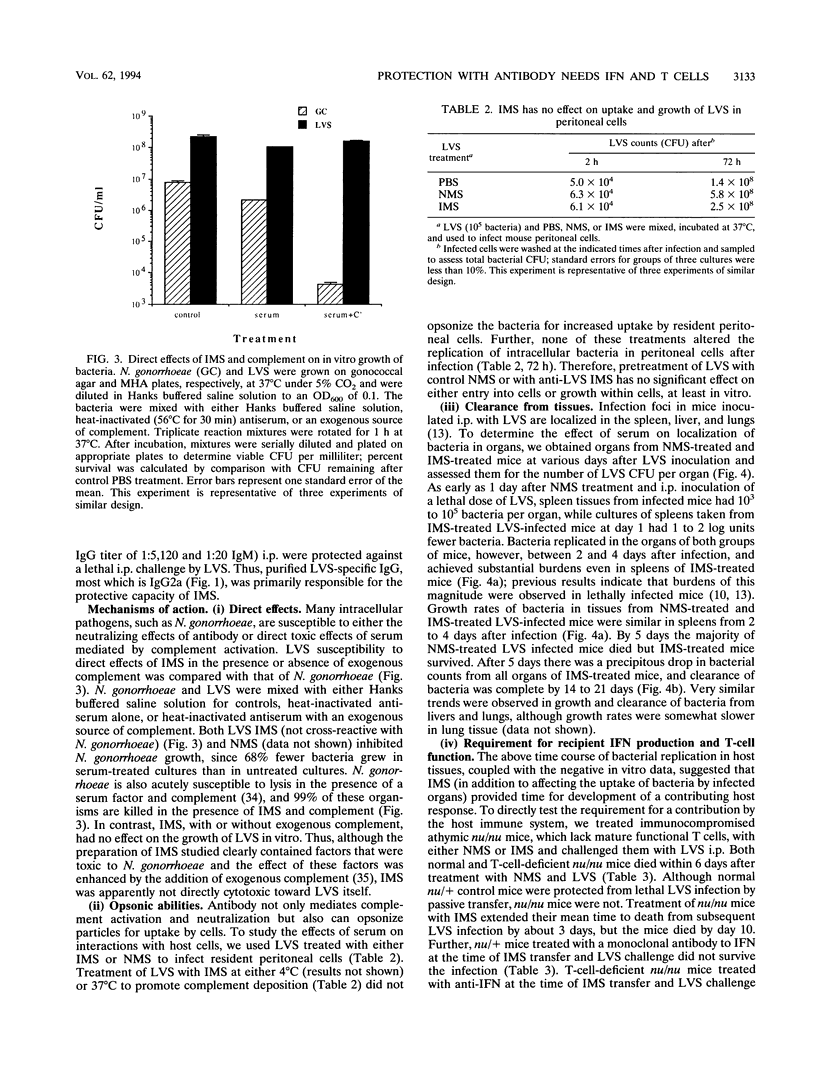
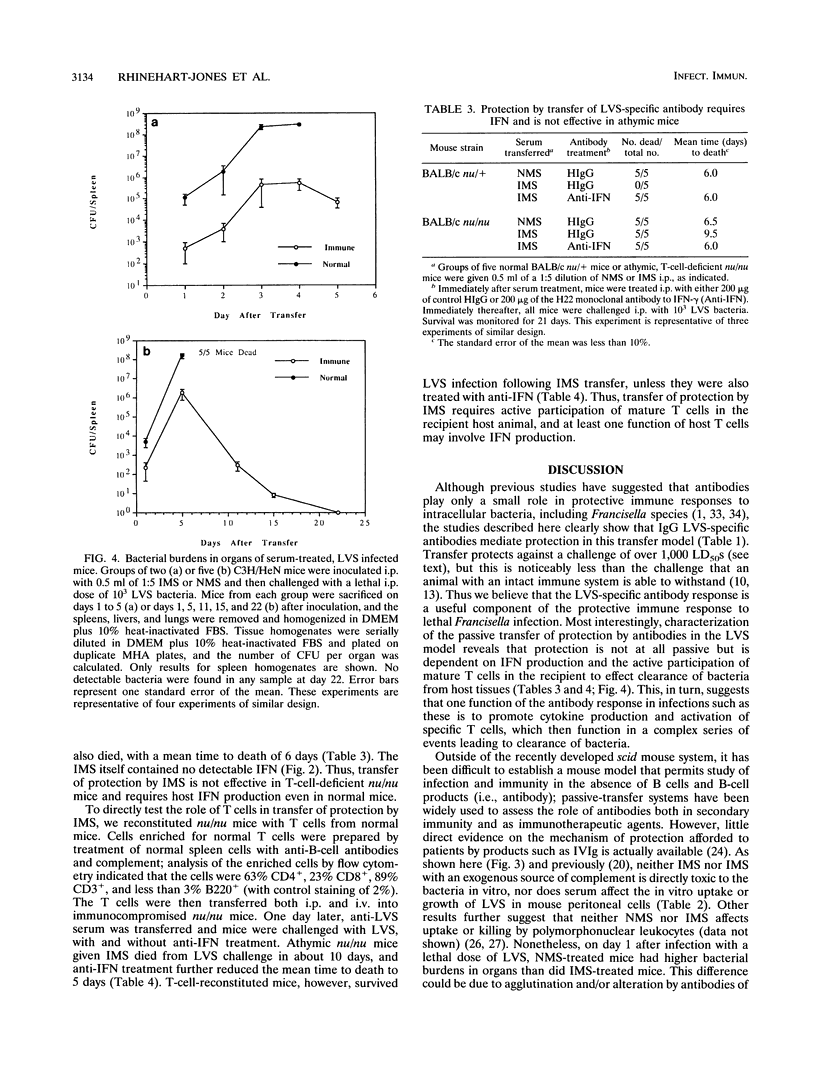
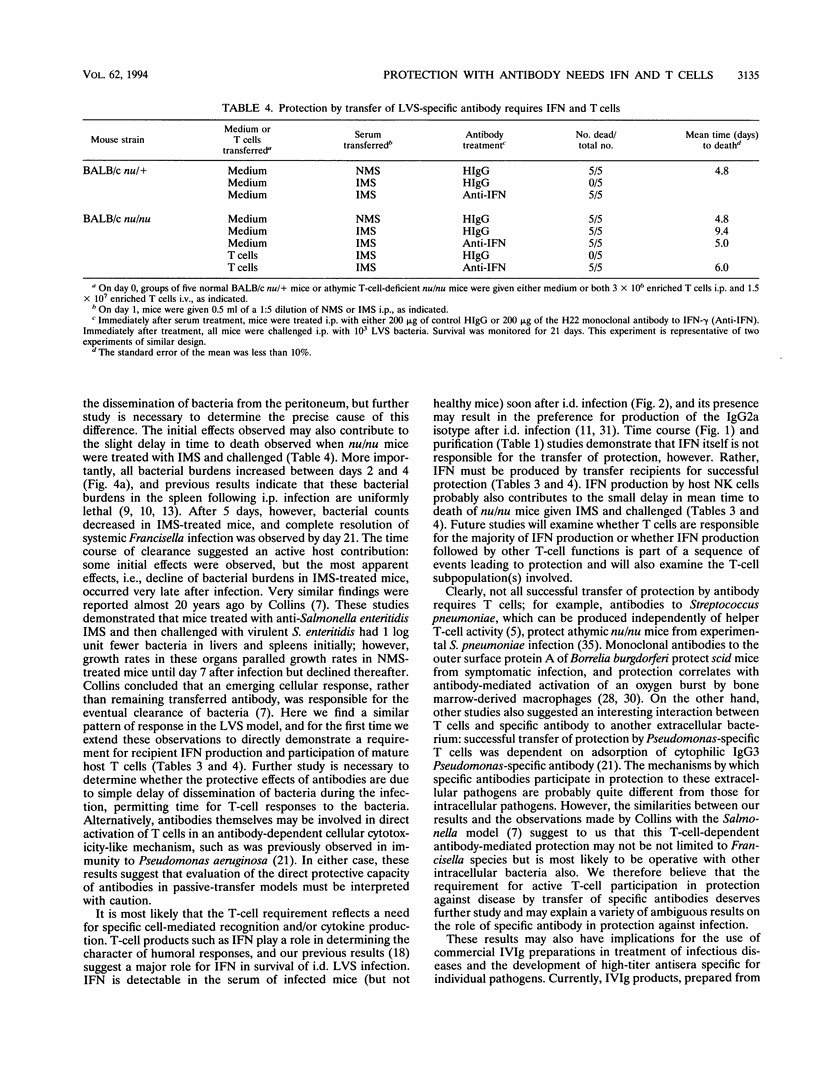
Both serum and spleen cells from mice immune to Francisella tularensis transfer protection to naive recipients. Here we characterize the mechanism of protection induced by transfer of immune mouse serum (IMS). IMS obtained 4 weeks after intradermal infection with 10(3) bacteria of the live vaccine strain (LVS) contained high levels of immunoglobulin G2 (IgG2a) and IgM (end point titers, 1:16,600 and 1:7,200, respectively) and little IgG1, IgG2b, or IgG3. LVS-specific antibodies were detected 5 days after intradermal infection, and reached peak levels by 2 weeks postinfection. Only sera obtained 10 days or more after sublethal infection, when IgG titers peaked, transferred protection against a challenge of 100 50% lethal doses (LD50s). Purified high-titer IgG anti-LVS antibody but not IgM anti-LVS antibody was responsible for transfer of protection against an intraperitoneal challenge of up to 3,000 LD50s. IMS had no direct toxic effects on LVS and did not affect uptake or growth of bacteria in association with peritoneal cells. One day after LVS infection, liver, spleen, and lung tissue from mice treated with IMS contained 1 to 2 log units fewer bacteria than did tissue from mice treated with normal mouse serum or phosphate-buffered saline. Between 2 and 4 days after infection, however, bacterial growth rates in tissues were similar in both serum-protected mice and unprotected mice. Bacterial burdens in IMS-treated, LVS-infected mice declined in infected tissues after day 5, whereas control animals died. This lag phase suggested that development of a host response was involved in complete bacterial clearance. In fact, transfer of IMS into normal recipients that were simultaneously treated with anti-gamma interferon and challenged with LVS did not protect mice from death. Further, transfer of IMS into athymic nu/nu mice did not protect against LVS challenge; protection was, however, reconstituted by transfer of normal T cells into nu/nu mice. Thus, "passive" transfer of protection against LVS with specific antibody is not passive but depends on a host T-cell response to promote clearance of systemic infection and protection against lethal disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN W. P. Immunity against tularemia: passive protection of mice by transfer of immune tissues. J Exp Med. 1962 Feb 1;115:411–420. doi: 10.1084/jem.115.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony L. S., Ghadirian E., Nestel F. P., Kongshavn P. A. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989 Dec;7(6):421–428. doi: 10.1016/0882-4010(89)90022-3. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Melish M. E., Hall R. T., Casto D. T., Vasan U., Givner L. B. Intravenous immune globulin for the prevention of nosocomial infection in low-birth-weight neonates. The Multicenter Group for the Study of Immune Globulin in Neonates. N Engl J Med. 1992 Jul 23;327(4):213–219. doi: 10.1056/NEJM199207233270401. [DOI] [PubMed] [Google Scholar]
- Baker C. N., Hollis D. G., Thornsberry C. Antimicrobial susceptibility testing of Francisella tularensis with a modified Mueller-Hinton broth. J Clin Microbiol. 1985 Aug;22(2):212–215. doi: 10.1128/jcm.22.2.212-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J. Regulation of magnitude of antibody response to bacterial polysaccharide antigens by thymus-derived lymphocytes. Infect Immun. 1990 Nov;58(11):3465–3468. doi: 10.1128/iai.58.11.3465-3468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley R. H., Schiff R. I. The use of intravenous immune globulin in immunodeficiency diseases. N Engl J Med. 1991 Jul 11;325(2):110–117. doi: 10.1056/NEJM199107113250207. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIGELSBACH H. T., DOWNS C. M. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961 Oct;87:415–425. [PubMed] [Google Scholar]
- Elkins K. L., Rhinehart-Jones T., Nacy C. A., Winegar R. K., Fortier A. H. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect Immun. 1993 Mar;61(3):823–829. doi: 10.1128/iai.61.3.823-829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins K. L., Winegar R. K., Nacy C. A., Fortier A. H. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb Pathog. 1992 Nov;13(5):417–421. doi: 10.1016/0882-4010(92)90085-3. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Fortier A. H., Polsinelli T., Green S. J., Nacy C. A. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun. 1992 Mar;60(3):817–825. doi: 10.1128/iai.60.3.817-825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier A. H., Slayter M. V., Ziemba R., Meltzer M. S., Nacy C. A. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991 Sep;59(9):2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givner L. B. Human immunoglobulins for intravenous use: comparison of available preparations for group B streptococcal antibody levels, opsonic activity, and efficacy in animal models. Pediatrics. 1990 Dec;86(6):955–962. [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity to mycobacteria. Res Microbiol. 1990 Sep-Oct;141(7-8):765–768. doi: 10.1016/0923-2508(90)90108-3. [DOI] [PubMed] [Google Scholar]
- Leiby D. A., Fortier A. H., Crawford R. M., Schreiber R. D., Nacy C. A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992 Jan;60(1):84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfgren S., Tärnvik A., Bloom G. D., Sjöberg W. Phagocytosis and killing of Francisella tularensis by human polymorphonuclear leukocytes. Infect Immun. 1983 Feb;39(2):715–720. doi: 10.1128/iai.39.2.715-720.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfgren S., Tärnvik A., Thore M., Carlsson J. A wild and an attenuated strain of Francisella tularensis differ in susceptibility to hypochlorous acid: a possible explanation of their different handling by polymorphonuclear leukocytes. Infect Immun. 1984 Feb;43(2):730–734. doi: 10.1128/iai.43.2.730-734.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham R. B., Pier G. B., Schreiber J. R. The role of cytophilic IgG3 antibody in T cell-mediated resistance to infection with the extracellular bacterium, Pseudomonas aeruginosa. J Immunol. 1991 Jan 1;146(1):316–320. [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Meltzer M. S., Buchmeier N. A., Schreiber R. D. Macrophage activation to kill Leishmania major: activation of macrophages for intracellular destruction of amastigotes can be induced by both recombinant interferon-gamma and non-interferon lymphokines. J Immunol. 1985 Nov;135(5):3505–3511. [PubMed] [Google Scholar]
- Portnoy D. A. Innate immunity to a facultative intracellular bacterial pathogen. Curr Opin Immunol. 1992 Feb;4(1):20–24. doi: 10.1016/0952-7915(92)90118-x. [DOI] [PubMed] [Google Scholar]
- Sandström G., Löfgren S., Tärnvik A. A capsule-deficient mutant of Francisella tularensis LVS exhibits enhanced sensitivity to killing by serum but diminished sensitivity to killing by polymorphonuclear leukocytes. Infect Immun. 1988 May;56(5):1194–1202. doi: 10.1128/iai.56.5.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Eichmann K., Modolell M., Museteanu C., Simon M. M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A. 1990 May;87(10):3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siber G. R. Immune globulin to prevent nosocomial infections. N Engl J Med. 1992 Jul 23;327(4):269–271. doi: 10.1056/NEJM199207233270409. [DOI] [PubMed] [Google Scholar]
- Simon M. M., Schaible U. E., Wallich R., Kramer M. D. A mouse model for Borrelia burgdorferi infection: approach to a vaccine against Lyme disease. Immunol Today. 1991 Jan;12(1):11–16. doi: 10.1016/0167-5699(91)90106-4. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- THORPE B. D., MARCUS S. PHAGOCYTOSIS AND INTRACELLULAR FATE OF PASTEURELLA TULARENSIS. 3. IN VIVO STUDIES WITH PASSIVELY TRANSFERRED CELLS AND SERA. J Immunol. 1965 Apr;94:578–585. [PubMed] [Google Scholar]
- Tärnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989 May-Jun;11(3):440–451. [PubMed] [Google Scholar]
- Wetzler L. M., Barry K., Blake M. S., Gotschlich E. C. Gonococcal lipooligosaccharide sialylation prevents complement-dependent killing by immune sera. Infect Immun. 1992 Jan;60(1):39–43. doi: 10.1128/iai.60.1.39-43.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A., Swift A. J. Host defense against the pneumococcus in T-lymphocyte-deficient, nude mice. Infect Immun. 1975 Nov;12(5):1222–1223. doi: 10.1128/iai.12.5.1222-1223.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



