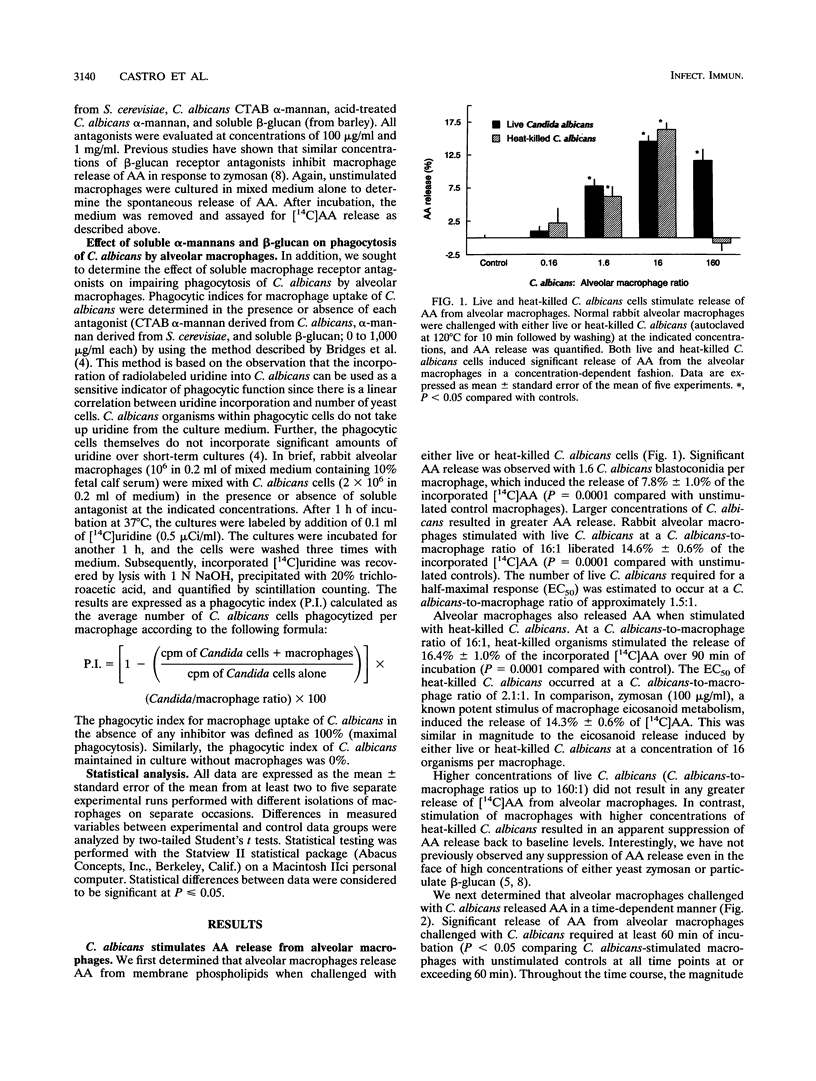
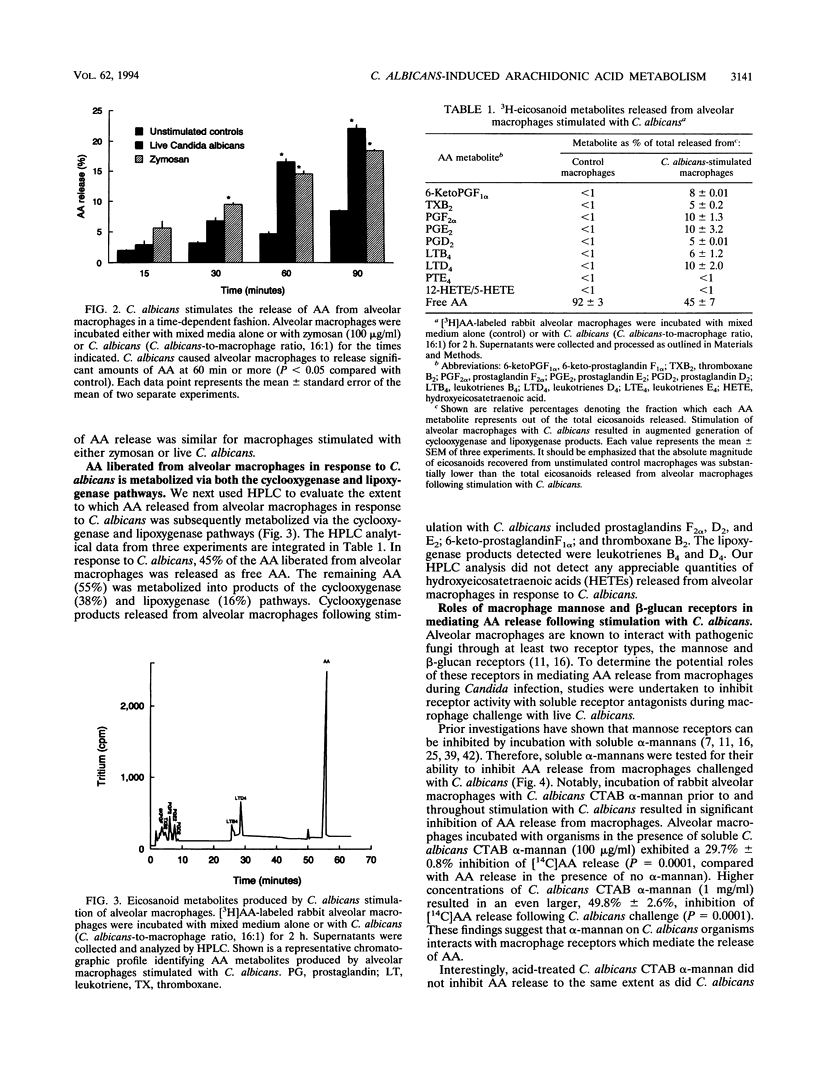
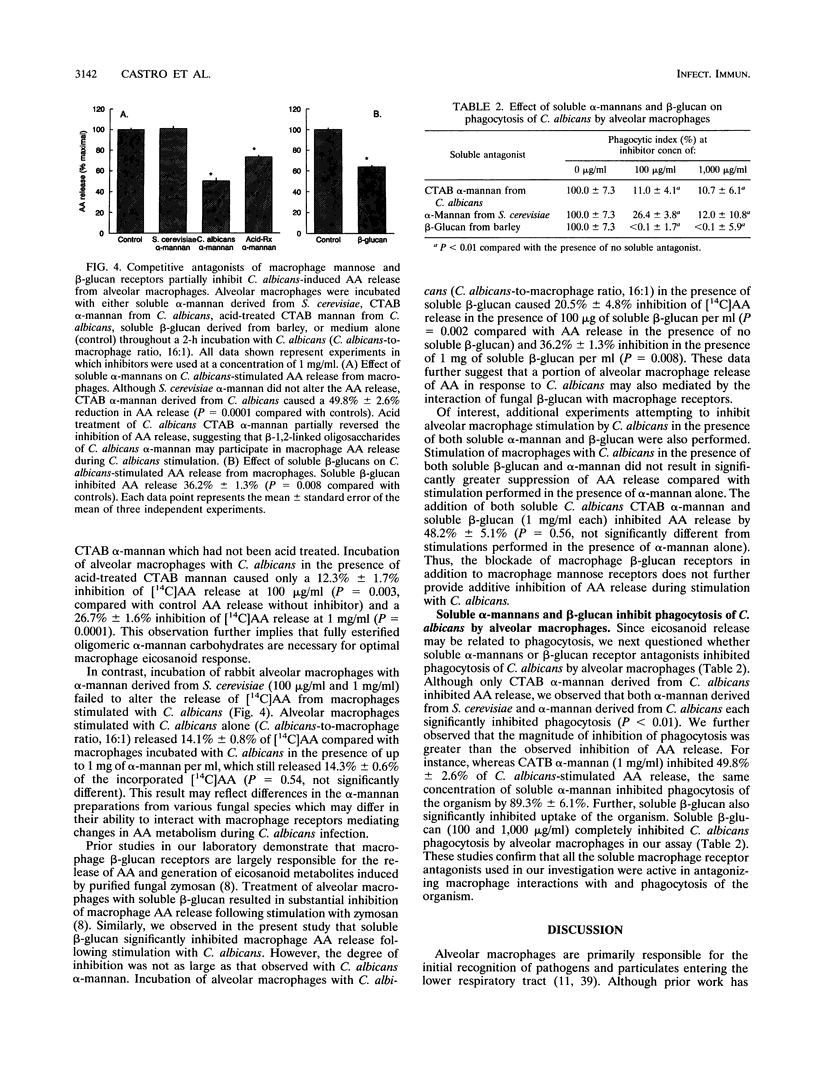
Abstract
Candida albicans is an increasingly important fungal pathogen. Alveolar macrophages respond to fungal components such as zymosan by releasing arachidonic acid (AA) and AA metabolites. However, few studies hypothesized that macrophages respond to C. albicans by releasing AA and generating AA metabolites as a consequence of interaction of mannose and beta-glucan receptors with fungal cell wall components. [14C]AA-labeled rabbit alveolar macrophages released AA following stimulation with either live or heat-killed C. albicans. High-pressure liquid chromatography analysis revealed that 55% of the AA released was metabolized via cyclooxygenase and lipoxygenase pathways. The metabolites consisted of prostaglandin E2, prostaglandin F2 alpha, 6-ketoprostaglandin F1 alpha, thromboxane B2, and leukotrienes B4 and D4. We further examined the roles of alpha-mannan and beta-glucan components of C. albicans in mediating these alterations of eicosanoid metabolism. Prior work in our laboratory has shown that soluble alpha-mannan and beta-glucan inhibit macrophage mannose and beta-glucan receptors, respectively. Incubation of alveolar macrophages with soluble alpha-mannan derived from C. albicans (1 mg/ml) resulted in 49.8% +/- 2.6% inhibition of macrophage AA release during stimulation with intact C. albicans (P = 0.0001 versus control). Macrophage AA release in response to C. albicans was also inhibited to a significant but lesser degree by soluble beta-glucan (36.2% +/- 1.3%; P = 0.008 versus control). These results indicate that C. albicans stimulates macrophage AA metabolism and that these effects are partly mediated by alpha-mannan and beta-glucan constituents of the fungus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balter M. S., Toews G. B., Peters-Golden M. Different patterns of arachidonate metabolism in autologous human blood monocytes and alveolar macrophages. J Immunol. 1989 Jan 15;142(2):602–608. [PubMed] [Google Scholar]
- Berton G., Gordon S. Modulation of macrophage mannosyl-specific receptors by cultivation on immobilized zymosan. Effects on superoxide-anion release and phagocytosis. Immunology. 1983 Aug;49(4):705–715. [PMC free article] [PubMed] [Google Scholar]
- Bonney R. J., Wightman P. D., Davies P., Sadowski S. J., Kuehl F. A., Jr, Humes J. L. Regulation of prostaglandin synthesis and of the selective release of lysosomal hydrolases by mouse peritoneal macrophages. Biochem J. 1978 Nov 15;176(2):433–442. doi: 10.1042/bj1760433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C. G., Dasilva G. L., Yamamura M., Valdimarsson H. A radiometric assay for the combined measurement of phagocytosis and intracellular killing of Candida albicans. Clin Exp Immunol. 1980 Nov;42(2):226–233. [PMC free article] [PubMed] [Google Scholar]
- Castro M., Morgenthaler T. I., Hoffman O. A., Standing J. E., Rohrbach M. S., Limper A. H. Pneumocystis carinii induces the release of arachidonic acid and its metabolites from alveolar macrophages. Am J Respir Cell Mol Biol. 1993 Jul;9(1):73–81. doi: 10.1165/ajrcmb/9.1.73. [DOI] [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. A beta-glucan inhibitable receptor on human monocytes: its identity with the phagocytic receptor for particulate activators of the alternative complement pathway. J Immunol. 1985 Apr;134(4):2588–2593. [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. Generation of leukotrienes by human monocytes upon stimulation of their beta-glucan receptor during phagocytosis. Proc Natl Acad Sci U S A. 1985 May;82(9):2751–2755. doi: 10.1073/pnas.82.9.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum T., Rohrbach M. S. Zymosan induces selective release of arachidonic acid from rabbit alveolar macrophages via stimulation of a beta-glucan receptor. FEBS Lett. 1992 Sep 7;309(2):119–122. doi: 10.1016/0014-5793(92)81077-y. [DOI] [PubMed] [Google Scholar]
- Edwards J. E., Jr Invasive candida infections--evolution of a fungal pathogen. N Engl J Med. 1991 Apr 11;324(15):1060–1062. doi: 10.1056/NEJM199104113241511. [DOI] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Lehrer R. I., Stiehm E. R., Fischer T. J., Young L. S. Severe candidal infections: clinical perspective, immune defense mechanisms, and current concepts of therapy. Ann Intern Med. 1978 Jul;89(1):91–106. doi: 10.7326/0003-4819-89-1-91. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Williams D. J., Koziel H., Armstrong M. Y., Warner A., Richards F. F., Rose R. M. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991 May 9;351(6322):155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- Filler S. G., Ibe B. O., Luckett P. M., Raj J. U., Edwards J. E., Jr Candida albicans stimulates endothelial cell eicosanoid production. J Infect Dis. 1991 Nov;164(5):928–935. doi: 10.1093/infdis/164.5.928. [DOI] [PubMed] [Google Scholar]
- Goldman R. Characteristics of the beta-glucan receptor of murine macrophages. Exp Cell Res. 1988 Feb;174(2):481–490. doi: 10.1016/0014-4827(88)90317-5. [DOI] [PubMed] [Google Scholar]
- Gopal P., Sullivan P. A., Shepherd M. G. Isolation and structure of glucan from regenerating spheroplasts of Candida albicans. J Gen Microbiol. 1984 May;130(5):1217–1225. doi: 10.1099/00221287-130-5-1217. [DOI] [PubMed] [Google Scholar]
- Hoffman O. A., Standing J. E., Limper A. H. Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-mediated mechanism. J Immunol. 1993 May 1;150(9):3932–3940. [PubMed] [Google Scholar]
- Holtzman M. J. Arachidonic acid metabolism. Implications of biological chemistry for lung function and disease. Am Rev Respir Dis. 1991 Jan;143(1):188–203. doi: 10.1164/ajrccm/143.1.188. [DOI] [PubMed] [Google Scholar]
- Hsueh W., Kuhn C., 3rd, Needleman P. Relationship of prostaglandin secretion by rabbit alveolar macrophages to phagocytosis and lysosomal enzyme release. Biochem J. 1979 Nov 15;184(2):345–354. doi: 10.1042/bj1840345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannace P. W., Lerman R. H., Santos J. I., Vitale J. J. Effects of oral soy phosphatidylcholine on phagocytosis, arachidonate concentrations, and killing by human polymorphonuclear leukocytes. Am J Clin Nutr. 1992 Sep;56(3):599–603. doi: 10.1093/ajcn/56.3.599. [DOI] [PubMed] [Google Scholar]
- Janusz M. J., Austen K. F., Czop J. K. Isolation of soluble yeast beta-glucans that inhibit human monocyte phagocytosis mediated by beta-glucan receptors. J Immunol. 1986 Nov 15;137(10):3270–3276. [PubMed] [Google Scholar]
- Kitz D. J., Stahl P. D., Little J. R. The effect of a mannose binding protein on macrophage interactions with Candida albicans. Cell Mol Biol. 1992 Jul;38(4):407–412. [PubMed] [Google Scholar]
- Kreofsky T., Schlager J. W., Vuk-Pavlović Z., Abraham R. T., Rohrbach M. S. Condensed tannin promotes the release of arachidonic acid from rabbit resident alveolar macrophages. Am J Respir Cell Mol Biol. 1992 Aug;7(2):172–181. doi: 10.1165/ajrcmb/7.2.172. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W. The role of arachidonic acid metabolites in mononuclear phagocytic cell interactions. Int J Dermatol. 1986 Mar;25(2):83–89. doi: 10.1111/j.1365-4362.1986.tb04543.x. [DOI] [PubMed] [Google Scholar]
- Lennartz M. R., Wileman T. E., Stahl P. D. Isolation and characterization of a mannose-specific endocytosis receptor from rabbit alveolar macrophages. Biochem J. 1987 Aug 1;245(3):705–711. doi: 10.1042/bj2450705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon F. L., Domer J. E. Chemical and enzymatic variation in the cell walls of pathogenic Candida species. Can J Microbiol. 1985 Jul;31(7):590–597. doi: 10.1139/m85-112. [DOI] [PubMed] [Google Scholar]
- Masur H., Rosen P. P., Armstrong D. Pulmonary disease caused by Candida species. Am J Med. 1977 Dec;63(6):914–925. doi: 10.1016/0002-9343(77)90546-0. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Characterization of the carbohydrate fragments obtained from Saccharomyces cerevisiae mannan by alkaline degradation. J Biol Chem. 1974 Dec 10;249(23):7679–7684. [PubMed] [Google Scholar]
- Nelson R. D., Shibata N., Podzorski R. P., Herron M. J. Candida mannan: chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin Microbiol Rev. 1991 Jan;4(1):1–19. doi: 10.1128/cmr.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHAFF H. J. CELL WALL OF YEASTS. Annu Rev Microbiol. 1963;17:15–30. doi: 10.1146/annurev.mi.17.100163.000311. [DOI] [PubMed] [Google Scholar]
- Podzorski R. P., Gray G. R., Nelson R. D. Different effects of native Candida albicans mannan and mannan-derived oligosaccharides on antigen-stimulated lymphoproliferation in vitro. J Immunol. 1990 Jan 15;144(2):707–716. [PubMed] [Google Scholar]
- Podzorski R. P., Herron M. J., Fast D. J., Nelson R. D. Pathogenesis of candidiasis. Immunosuppression by cell wall mannan catabolites. Arch Surg. 1989 Nov;124(11):1290–1294. doi: 10.1001/archsurg.1989.01410110044009. [DOI] [PubMed] [Google Scholar]
- Rohrbach M. S., Kreofsky T., Rolstad R. A., Russell J. A. Tannin-mediated secretion of a neutrophil chemotactic factor from alveolar macrophages. Potential contribution to the acute pulmonary inflammatory reaction associated with byssinosis. Am Rev Respir Dis. 1989 Jan;139(1):39–45. doi: 10.1164/ajrccm/139.1.39. [DOI] [PubMed] [Google Scholar]
- Rose H. D., Sheth N. K. Pulmonary candidiasis. A clinical and pathological correlation. Arch Intern Med. 1978 Jun;138(6):964–965. doi: 10.1001/archinte.138.6.964. [DOI] [PubMed] [Google Scholar]
- San-Blas G. The cell wall of fungal human pathogens: its possible role in host-parasite relationships. Mycopathologia. 1982 Sep 17;79(3):159–184. doi: 10.1007/BF01837196. [DOI] [PubMed] [Google Scholar]
- Saxena A., Hammer C. F., Cihlar R. L. Analysis of mannans of two relatively avirulent mutant strains of Candida albicans. Infect Immun. 1989 Feb;57(2):413–419. doi: 10.1128/iai.57.2.413-419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A., McElhaney-Feser G. E., Cihlar R. L. Mannan composition of the hyphal form of two relatively avirulent mutants of Candida albicans. Infect Immun. 1990 Jul;58(7):2061–2066. doi: 10.1128/iai.58.7.2061-2066.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd V. L., Campbell E. J., Senior R. M., Stahl P. D. Characterization of the mannose/fucose receptor on human mononuclear phagocytes. J Reticuloendothel Soc. 1982 Dec;32(6):423–431. [PubMed] [Google Scholar]
- Sibille Y., Reynolds H. Y. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990 Feb;141(2):471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Silverstein S. C. Phagocytosis of unopsonized zymosan by human monocyte-derived macrophages: maturation and inhibition by mannan. J Leukoc Biol. 1985 Nov;38(5):655–658. doi: 10.1002/jlb.38.5.655. [DOI] [PubMed] [Google Scholar]
- Stahl P. D. The macrophage mannose receptor: current status. Am J Respir Cell Mol Biol. 1990 Apr;2(4):317–318. doi: 10.1165/ajrcmb/2.4.317. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J Cell Biol. 1983 Jan;96(1):160–166. doi: 10.1083/jcb.96.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Fukazawa Y. Immunochemical characterization of Candida albicans cell wall antigens: specific determinant of Candida albicans serotype A mannan. Microbiol Immunol. 1982;26(5):387–402. doi: 10.1111/j.1348-0421.1982.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Tojo M., Shibata N., Kobayashi M., Mikami T., Suzuki M., Suzuki S. Preparation of monoclonal antibodies reactive with beta-1,2-linked oligomannosyl residues in the phosphomannan-protein complex of Candida albicans NIH B-792 strain. Clin Chem. 1988 Mar;34(3):539–543. [PubMed] [Google Scholar]
- Wolf J. E., Massof S. E., Peters S. P. Alterations in murine macrophage arachidonic acid metabolism following ingestion of nonviable Histoplasma capsulatum. Infect Immun. 1992 Jul;60(7):2559–2564. doi: 10.1128/iai.60.7.2559-2564.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


