Abstract
Cannabis use is common, controversial and its clinical toxicology is likely under-recognised. A 56-year-old man presented with disabling shortness of breath. He smoked up to 7 g cannabis daily for 25 years (maximum 63 875 g) plus large amounts of hashish oil. Chest x ray suggested giant bullae. CT of the chest revealed over 40 bullae, the largest being 11 cm in diameter. Osteoporosis with multiple vertebral crush fractures was noted on plain films and bone densitometry (t=−3.19). His dental health was poor. Hypertension, complicated by a large occipital stroke was shown by CT of the brain, and increased vascular age (69.8 years) found by pulse wave analysis. The case is significant as it indicates the potential severity of cannabis lung damage and suggests that significant cannabis exposure may cause osteoporosis and accelerated vascular ageing. The association of alveolar destruction, bone lysis and destruction of arterial elastic laminae suggest possible underlying mechanisms such as tissue proteinase activation, immunomodulation or stem cell impairment.
BACKGROUND
Cannabis use is widespread and increasing in many places including the UK and The Netherlands.1 The high prevalence of cannabis use has drawn attention from learned colleges, particularly in respiratory medicine,2,3 which has had the effect of elucidating cannabis-related respiratory toxicology4 including upper and lower respiratory tract malignancy.5,6 Similar advances have been made in understanding the neuropsychiatric toxicology of the cannabinoids, which is also a subject moving through a great period of clarification.7,8 Adverse findings have also been reported in other systems including osteoporosis9 and dental/periodontal disease.10 This latter finding is of great concern due to the association of aggressive periodontal disease with systemic ill health, reduced longevity and multisystem disease.11,12 Other causes for concern exist with its now demonstrated effects as a gateway drug to other drug use,13 because of its implications with regard to impaired vehicle driver performance,14 and greatly diminished developmental trajectory.15
The present case is important as it demonstrates the extreme severity of cannabis-related lung disease, the multisystem nature of cannabis-related dysfunction and some implications for population health of likely dose-related cannabis toxicity.
CASE PRESENTATION
Our patient was a 56-year-old known hypertensive man who presented with a history of weakness, tiredness and exhaustion with minimal exercise. His exercise tolerance was 50 m. He had smoked only up to six cigarettes daily for two 6-month periods. His cannabis consumption however had been heavy, using from 1–7 g daily mixed with a little tobacco (up to 10 cigarettes) in addition to large quantities of hashish oil, from the ages of 22 to 47 years. This represents an approximate maximum of 63 875 gram-years of cannabis use in addition to hashish. He had smoked large cannabis joints, had often used hashish oil through a chillum and routinely performed the Valsalva manoeuvre while smoking. He had only injected heroin five times in his life. He rarely drank alcohol. He had been largely unemployed and had experienced great difficulty holding down work in view of his poor physical health. He had no relevant occupational or family history.
Apart from his mild hypertension he had no major medical illnesses. His only medication was telmisartan 80 mg daily. On physical examination he was 173 cm, 78 kg (body mass index (BMI) 26.1), his blood pressure was 160/106 with a regular pulse of 82 beats/min, but there was no other abnormality on examination of the general, respiratory or neurological systems. His dental examination showed large cavities replacing more than 75% of the visible portion of the teeth in six of the eight posterior molars, which had been restored with amalgam, which the patient attributed to his strong sweet preference while “stoned”.
INVESTIGATIONS
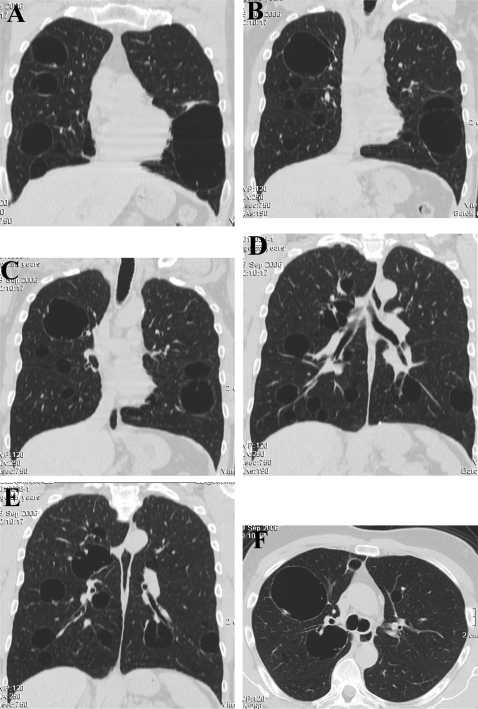
A chest x ray was performed, which illustrated giant bullae, 2 of which had diameters of 9 cm and 11 cm, osteoporosis and vertebral crush fractures of the 6th, 8th and 12th thoracic vertebrae. A CT scan of chest was performed (fig 1) which showed dozens of cysts in the lung parenchyma. Complex lung function studies were performed which showed that the carbon monoxide transfer factor was only mildly reduced at 81% of predicted. Large airways function was preserved with forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) above 94% predicted values and FEV1/VC=86%. Small airways function was dramatically reduced with the maximum mid expiratory flow rate (MMEFR)25–75 45% of predicted. A histamine challenge test revealed a 20% fall in FEV1 (PC20) of 6 mg/ml, demonstrating reversible airways disease.
Figure 1.
CT of the chest. Coronal (A–E) and axial (F) sections through the chest giant bullae.
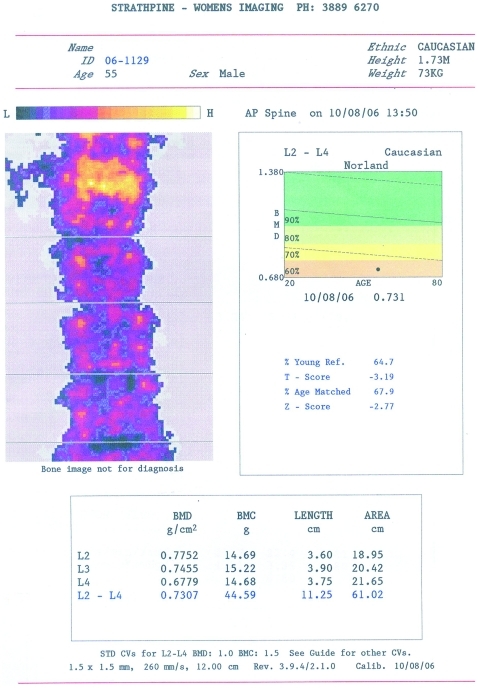
Bone mineral density was performed which showed osteopaenia in the osteoporotic range with a T score of −3.19 compared to a healthy young normal population and a Z score of −2.77 compared to age-matched normal controls in the lumbar spine (fig 2), with similar findings at the femoral neck. An abdominal CT scan showed three simple 1–2 cm diameter cysts in the liver and the right kidney but none elsewhere. Evaluation of the kidneys and renal arteries by ultrasound, CT angiography, MRI and magnetic resonance angiography revealed no evidence of renal artery stenosis or renal disease. The α1 anti-trypsin level was normal. On the basis of these findings a respiratory consultant felt that the likely diagnosis was multiorgan cystic disease exacerbated in the lungs by the smoking of tobacco and cannabis, and asthma. Therefore the patient was asked to report and seek treatment early for any respiratory infections and given a combination prophylactic eformoterol/budesonide.
Figure 2.
Bone mineral densitometry of the lumbar spine.
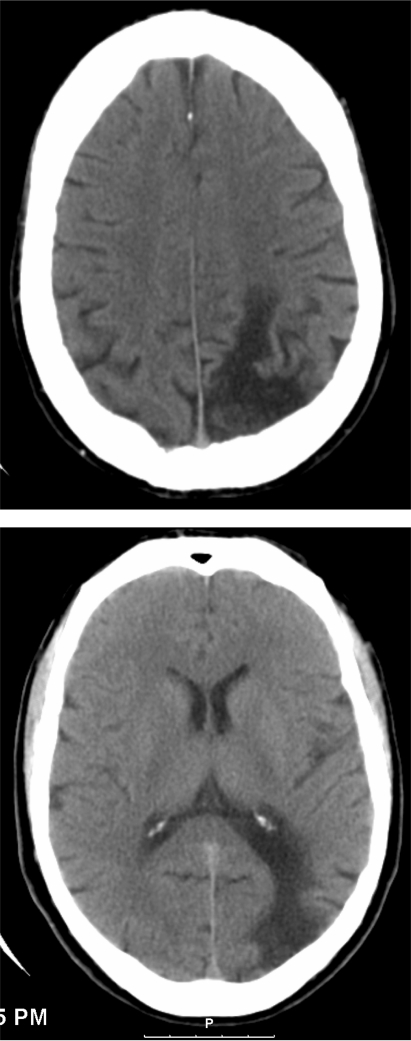
The patient returned again with intermittent profound lethargy, confusion and fainting. An electroencephalograph (EEG) was normal. A CT scan of his brain was performed (fig 3), which showed a large defect in the watershed area in the left parieto-occipital cerebral cortex and a lacunar typically hypertensive infarct near the left frontal lobe foramen semiovale. A hepatitis C test was positive.
Figure 3.
CT of the brain showing large parietal watershed infarct.
After treatment his central vascular impedance (aortic stiffness) was studied with the SphygmoCor system (AtCor Medical, West Ryde, Australia) and was found to be within the published parameters.16 His subendocardial perfusion viability ratio was depressed at 107.6, a level 1.7 SDs below the applicable age-matched and sex-matched mean. While his chronological age at the time of study was 57.2 years his biological vascular age was significantly elevated at 69.8 years.
DIFFERENTIAL DIAGNOSIS
Giant cystic lung disease, emphysema, hypertension, cerebrovascular accident related to hypertension with/without a cannabis-associated coagulopathy, edentulous, osteoporosis, depression (largely resolved).
TREATMENT
The patient was clinically much improved on a combination eformoterol/budesonide turbohaler and perindopril/indapamide.
OUTCOME AND FOLLOW-UP
The patient’s exercise tolerance increased to 500 m. He enjoyed improved community integration, and was drug free. However, the patient died 6 months prior to the publication of this report.
DISCUSSION
By performing the Valsalva manoeuvre during smoking, and by inducing airway inflammation and reducing airway conductance,17–19 the smoking of cannabis has been noted to increase the severity of cystic disorders of the lung by the British Lung Foundation3 and the Thoracic Society of Australia and New Zealand.2 Cannabis smoking has been found to be associated with chronic bronchitis, large airways inflammation, bronchial hyper-reactivity,19 alveolitis20 emphysema21,22 and cyst formation,19,23 as well as preneoplastic changes in upper and lower airways. Malignancies have been noted in upper and lower6 airways. Airway inflammation can increase the resistance to airflow. Because of Laplace’s law the tension in the bulla wall is related to the fourth power of the resistance pressure to airflow. Clearly such tendencies are made worse by the common technique of cannabis smoking involving deep inhalations and prolonged breath holding. The present case clearly demonstrates the severity to which such changes can develop.
Chronic lung disease and emphysema are known to be associated with systemic hyperimmune states and chronic bone loss. Chronic obstructive pulmonary disease (COPD) is known to affect cellular and humoural immunity. Cannabinoid receptors occur in the hypothalamus and in bone and are thought to interfere with bone formation by both hormonal mechanisms, by way of stimulation of proinflammatory cytokines, immunosuppression, nutritional compromise and physical inactivity.
Cannabis has also been associated with thrombotic and infarctive states in heart, kidney and cerebral tissues. A proinflammatory state has been suggested to be implicated in the pathogenesis of atherosclerotic and osteoporotic disease. Associations between osteoporosis, declining FEV1, cognition and emphysematous lung disease have been found in several clinical and preclinical models of accelerated ageing,19 and there is evidence that cannabis is associated with poor dentition.10 In mice cannabis has been associated with osteoporosis.9
It should be noted that cannabis has been associated with greatly increased rates of oxyradical flux rating as high as 1018 per puff of cannabis smoke. Oxyradicals have also been identified on binding to the type 1 cannabinoid receptor at the cell surface, by mitochondrial uncoupling and by direct toxicity to DNA with guanine oxidation. Despite detailed investigation a causal link between cannabis consumption and the development of hypertension in our patient was not established. The likely mechanism of the strokes in this patient was cerebrovascular accident related in part to cannabis and tobacco use and hypertension.
The association of dental disease with these changes is fascinating by virtue of its association with appetite stimulation and perturbation in terms of preference for sweet foods, and in relation to its association with immunological stimulation and stem cell inhibition. One of the classic molecular pathways of erosive dental disease is immune: infection-stimulated tissue proteinase activation. Evidence of such proteinase stimulation may be seen in this patient’s bones, teeth, great vessels, lungs and brain after his stroke. Although his central vascular compliance was normal after treatment, it was likely less so prior to adequate medical therapy. His vascular age remained elevated despite adequate antihypertensive treatment.
Although this patient did not describe evidence of overt mental disease, he did describe anhedonia and a period of sustained withdrawal from normal social and employment interaction throughout his prolonged period of dependency. At the societal level, the implications of prolonged welfare dependency are substantial.
There is no suggestion that the existence of the renal cysts was cannabis related.
This case underscores the severity and multiplicity of cannabis-related toxicopathologies, the parallel existence of hypodense lungs, brain and bones in association with hypertension and vascular ageing. Major current themes in the pathogenesis of COPD (emphysema, atherosclerosis, erosive dental disease and osteoporosis) include tissue proteinase activation, cytokine stimulation and stem cell impairment, which in turn are frequently based on increased rates of systemic inflammation and oxyradical generation. Their concurrence in this patient suggests that such processes may be acting systemically to induce severe multiorgan dysfunction and an accelerated pattern of ageing. This interesting possibility would appear to merit further research. Cases such as the present one involving exposure to particularly high doses may imply that any such processes might be dose related, and this possibility would clearly carry important public health implications. In the context of dose-related pathogenesis and increasingly widespread cannabis consumption such cases have major public health implications and provide an important stimulus for the research community to further explore relevant molecular toxicological mechanisms.
LEARNING POINTS
Cannabis lung disease can become very severe. Indeed, giant lung cysts can form in predisposed individuals.
Cannabis can affect other body systems such as the cardiovascular system, the bones, the brain and the kidneys.
There is a need to consider multisystem impacts in patients presenting with significant cannabis exposure.
The impacts of cannabis-related mental disorders, such as depression, can be far reaching and severe for lifestyle and life path.
The presence of multisystem disorders suggests that common underlying pathophysiological pathways may be implicated in several body systems. In view of the widespread use of cannabis, further investigation and elucidation in this area would appear to be a research priority.
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Cannabis: introduction: the changing picture of cannabis in Europe. http://www.emcdda.europa.eu/html.cfm/index41524EN.html (accessed 25 November 2007) [Google Scholar]
- 2.Taylor DR, Hall W. Respiratory health effects of cannabis: position statement of the Thoracic Society of Australia and New Zealand. Intern Med J 2003; 33: 310–3 [DOI] [PubMed] [Google Scholar]
- 3.Foundation British Lung. Cannabis: a smoking gun. Vol. 1 London, UK: British Lung Foundation, 2005 [Google Scholar]
- 4.Brambilla C, Cannabis Colonna M: the next villain on the lung cancer battlefield? Eur Respir J 2008; 31: 227–8 [DOI] [PubMed] [Google Scholar]
- 5.Zhang ZF, Morgenstern H, Spitz MR, et al. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev 1999; 8: 1071–8 [PubMed] [Google Scholar]
- 6.Aldington S, Harwood M, Cox B, et al. Cannabis use and risk of lung cancer: a case-control study. Eur Respir J 2008; 31: 280–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007; 370: 319–28 [DOI] [PubMed] [Google Scholar]
- 8.Nordentoft M, Hjorthoj C. Cannabis use and risk of psychosis in later life. Lancet 2007; 370: 293–4 [DOI] [PubMed] [Google Scholar]
- 9.Idris AI, van’t Hof RJ, Greig IR, et al. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med 2005; 11: 774–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson WM, Poulton R, Broadbent JM, et al. Cannabis smoking and periodontal disease among young adults. JAMA 2008; 299: 525–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desvarieux M, Demmer RT, Rundek T, et al. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Stroke 2003; 34: 2120–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med 2007; 356: 911–20 [DOI] [PubMed] [Google Scholar]
- 13.Fergusson DM, Boden JM, Horwood LJ. Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction 2006; 101: 556–69 [DOI] [PubMed] [Google Scholar]
- 14.Kelly E, Darke S, Ross J. A review of drug use and driving: epidemiology, impairment, risk factors and risk perceptions. Drug Alcohol Rev 2004; 23: 319–44 [DOI] [PubMed] [Google Scholar]
- 15.Patton GC, Coffey C, Lynskey MT, et al. Trajectories of adolescent alcohol and cannabis use into young adulthood. Addiction 2007; 102: 607–15 [DOI] [PubMed] [Google Scholar]
- 16.McEniery CM, Yasmin, Hall IR, Qasem A, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo–Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005; 46: 1753–60 [DOI] [PubMed] [Google Scholar]
- 17.Taylor DR, Fergusson DM, Milne BJ, et al. A longitudinal study of the effects of tobacco and cannabis exposure on lung function in young adults. Addiction 2002; 97: 1055–61 [DOI] [PubMed] [Google Scholar]
- 18.Taylor DR, Poulton R, Moffitt TE, et al. The respiratory effects of cannabis dependence in young adults. Addiction 2000; 95: 1669–77 [DOI] [PubMed] [Google Scholar]
- 19.Aldington S, Williams M, Nowitz M, et al. The effects of cannabis on pulmonary structure, function and symptoms. Thorax 2007; 62: 1058–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris RR. Human pulmonary histopathological changes from marijuana smoking. J Forensic Sci 1985; 30: 345–9 [PubMed] [Google Scholar]
- 21.Beshay M, Kaiser H, Niedhart D, et al. Emphysema and secondary pneumothorax in young adults smoking cannabis. Eur J Cardiothorac Surg 2007; 32: 834–8 [DOI] [PubMed] [Google Scholar]
- 22.Luque MA, 3rd, Cavallaro DL, Torres M, et al. Pneumomediastinum, pneumothorax, and subcutaneous emphysema after alternate cocaine inhalation and marijuana smoking. Pediatr Emerg Care 1987; 3: 107–9 [DOI] [PubMed] [Google Scholar]
- 23.Henquet C, Krabbendam L, Spauwen J, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ 2005; 330: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]