Abstract
Isolated native tricuspid valve endocarditis (TVE) in non-intravenous drug users is a very rare condition. We describe an unusual presentation of Enterococcus faecalis TVE associated with spondylodiscitis, positive cytoplasmic antineutrophil cytoplasmic antibodies and antiproteinase-3 antibodies vasculitic rash in an otherwise healthy patient with no history of intravenous drug use or underlying cardiac abnormalities.
A high index of clinical suspicion is required in patients presenting with unusual features and pyrexia of unknown origin. Simple tests including serial blood cultures and echocardiography may help to establish the correct diagnosis and commence appropriate treatment.
Background
The incidence of infective endocarditis (IE) in the general population is approximately 1.7–6.2 cases per 100 000 patient years with more frequent occurrence in at-risk cohorts such as intravenous drug users (150–2000 cases per 100 000 patient years).1 Tricuspid valve endocarditis (TVE) accounts for 5–10% of all cases of IE, but is more frequent in intravenous drug users, comprising up to 70% of all cases of IE.2 3
To date, there are only few studies that report association of IE with positive antiproteinase-3 antibody (anti-PR3). Concomitant existence of IE with spondylodiscitis has been more widely described in the literature. This case illustrates important teaching points.
Case presentation
A 65-year-old Caucasian male was referred to our hospital with a 6-week history of generalised non-pruritic skin rash and a 4-month history of back pain. Two weeks prior to admission, he visited his family doctor and was diagnosed with musculoskeletal back pain and an allergic skin rash. His medical history included ischaemic heart disease and lumbar spondylosis.
Physical examination was initially unremarkable, except for a non-blanching, well-circumscribed, purpuric rash mainly on the lower limbs and localised tenderness along the lower thoracic and lumbar spine. A working diagnosis of cryoglobulinaemic vasculitis was made.
Investigations
Initial investigations revealed haemoglobin 9.5 g/dl, mean corpuscular volume 78 fl, white blood cell count 9.4×109/l, platelets 173×109/l, negative urine dipstick and normal renal function. C reactive protein (CRP) was raised to 76 mg/l (normal <10).
Cytoplasmic antineutrophil cytoplasmic antibodies (cANCA) were positive (1:320), and anti-PR3 were detected at a titre of 24 IU/ml (normal <6). Cryoglobulins were also positive, but antinuclear antibodies and antibodies to extractable nuclear antigens (Ro, La, Sm, U1RNP, RNP70, CENP, Jo-1, Scl-70) were negative. Tests for hepatitis B antigen, hepatitis C antibodies and HIV antibodies were negative. The patient also underwent skin biopsy, which did not show any features of cryoglobulinaemia or leukocytoclastic vasculitis.
MRI of the thoraco-lumbar spine, requested to investigate back pain, revealed abnormal fluid collection in the L2/3, L3/4, L5/S1 discs with inflammatory changes in the adjacent end plates and further mild changes at T8/9 level suggestive of multifocal infective spondylodiscitis (figure 1).
Figure 1.
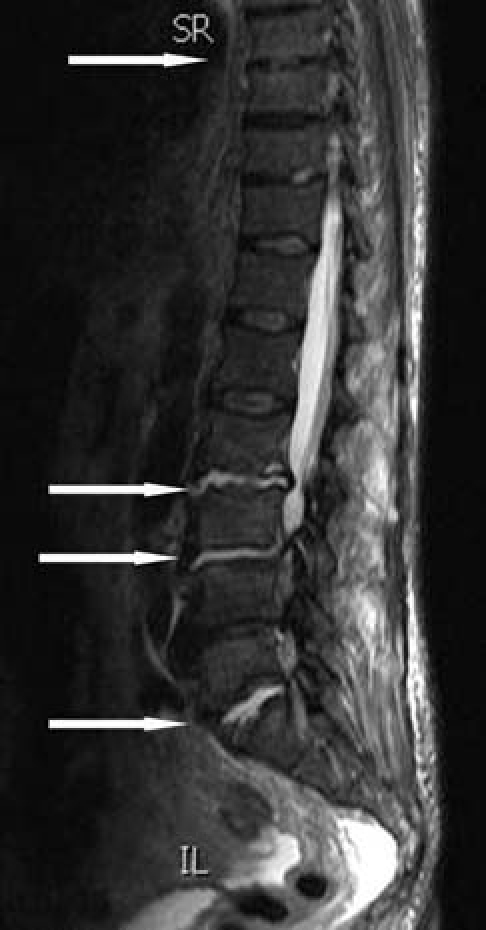
MRI of the spine showing abnormal fluid collection in the L2/3, L3/4, L5/S1 discs with inflammatory changes in the adjacent end plates with further mild changes at T8/9 level, in keeping with multifocal early infective spondylodiscitis in the lower thoracic and lumbar spine. Spondylodiscitic changes are indicated by arrows.
During hospitalisation, the patient developed pyrexia and three sets of blood cultures grew Enterococcus faecalis. Further clinical examination demonstrated splinter haemorrhages in three finger nails. Subsequently, transthoracic echocardiogram (TTE) and transoesophageal echocardiogram (TOE) were performed. Both revealed large tricuspid valve vegetations with severe tricuspid regurgitation associated with destruction of the septal leaflet (figure 2). TOE was performed to provide a more detailed anatomical assessment because despite appropriate antimicrobial therapy, the patient initially did not respond well to treatment and suffered from persistent fever and raised inflammatory markers. In addition, he had severe tricuspid regurgitation on TTE and TOE was needed for surgical planning.
Figure 2.
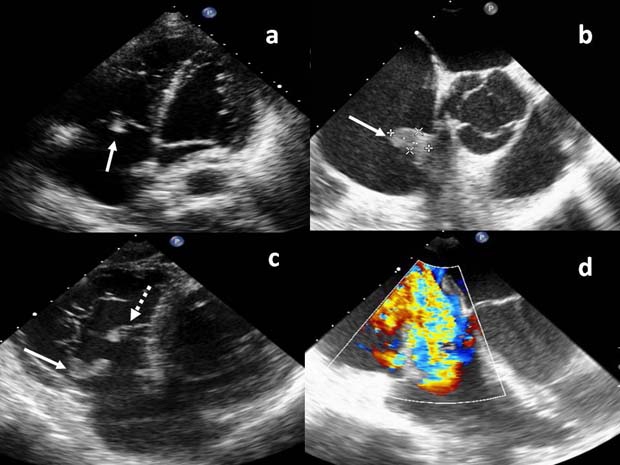
Transoesophageal echocardiogram showing: (A, B) tricuspid valve vegetation (solid arrows), (C) damaged septal leaflet (broken arrow) and (D) severe tricuspid regurgitation.
There was no obvious source of endogenous bacteraemia but in view of positive cultures for E faecalis and microcytic anaemia, the patient was investigated for colonic malignancy. Contrast CT scan of abdomen and pelvis, tumour markers including carcino-embryonic antigen, total hCG, α-fetoprotein, and carbohydrate antigen 19.9 (CA 19.9) and colonoscopy were normal.
Treatment
Initial treatment included intravenous amoxicillin and gentamicin for 3 weeks, followed by vancomycin for further 2 weeks and linezolid for a week. The antimicrobial agents were changed as the patient developed an itchy urticarial rash with peripheral eosinophilia, caused most likely by drug hypersensitivity reactions.
Outcome and follow-up
The patient's hospitalisation was further complicated by Pseudomonas aeruginosa (positive sputum cultures) pneumonia confirmed on chest x-ray, but overall he made a good recovery. Repeated blood cultures showed no growth and repeated TTE showed no changes. Repeated cANCA titres after the course of antibiotics were 1:40.
The patient's vasculitic skin lesions cleared gradually without recurrence. His back pain, which was initially very severe, also improved significantly during the 8-week hospitalisation. On discharge from hospital, his mobility was back to normal and he was using simple pain killers as required. CRP also normalised. Out-patient coronary angiography showed mild-to-moderate right coronary disease, that was not flow-limiting, and the patient eventually had successful elective tricuspid valve repair without the need for bypass surgery of the coronary arteries.
Discussion
Identification of isolated TVE in the absence of predisposing factors and history of intravenous drug use may be challenging. It is a rare condition, and as shown in a Canadian study on 135 cases, isolated native TVE was reported in 5% of non-drug users.4 A Finnish study of 326 IE cases showed that fever was the commonest presentation (75%), back pain was recorded only in 12% and skin lesions in less than 5% of cases, suggesting that by using these criteria, there would have been a low index of clinical suspicion for IE in this patient.5 In fact, it took 18 weeks before diagnosis of IE could be made and for treatment to be commenced. Previous reports indicate that the average duration of TVE before diagnosis might be as long as 9 months.6
Pulmonary presentation (pneumonia or septic pulmonary embolism) has been described in several studies as the predominant feature of TVE. Although our patient did develop P aeruginosa chest infection, it was late during hospitalisation and unlikely to be related to the endocarditis. In our case, there was no clear evidence of ‘tricuspid syndrome’ (recurrent pulmonary events, anaemia and microscopic haematuria) associated with TVE as described by Nandakumar et al.6
Diagnosis of IE should not be delayed and high index of clinical suspicion is required. TTE is an easily accessible and a non-invasive study that remains the first-choice modality to diagnose native valve IE. Although TTE has excellent specificity for vegetations up to 98%, it may be inadequate in up to 20% of patients because of obesity, chronic obstructive pulmonary disease or chest wall deformities; the overall sensitivity for vegetations may be less than 60%.1 TOE has better resolution and multiple study planes, thereby increasing sensitivity for detecting vegetations to 90–100%.1 After initial TTE and diagnosis of TVE, TOE was also required for further assessment of possible IE extension and abscess formation in view of poor initial response to antimicrobial treatment. TOE is more sensitive than TTE for detecting perivalvular extension of IE and the presence of a myocardial abscess. It also provides more detailed valvular anatomy when valvular surgery needs to be considered.7 Valve surgery may need to be done earlier if there is haemodynamic decompensation due to acute valvular regurgitation, persistent embolic phenomenon, persistent fever and bacteraemia despite appropriate antibiotic treatment, or development of abscess or fistulae caused by local spread of infection.8
Our patient presented with two extracardiac manifestations of TVE including spondylodiscitis and positive PR3-ANCA. Spondylodiscitis and E faecalis bacteraemia are known to have higher frequency of concomitant IE than spondylodiscitis caused by other bacteria. It is suggested that an echocardiogram be performed in every patient with streptococcal or enterococcal bacteraemia presenting with spondylodiscitis.9
Patients presenting with anti-PR3 positive vasculitis and subsequently diagnosed with IE have been described previously, showing a reduction of cANCA titres after commencement of antibiotics, without the need for corticosteroid therapy as in our patient.10 11 Chronic infection may mimic ANCA-associated vasculitides (AAV) as shown in a study by Bonaci-Nikolic et al where for 5.1 years the authors had followed up a group of patients with AAV and the PR3 and myeloperoxidase-ANCA were found to be positive with proven bacterial or viral infections. The most common infections that mimicked AAV were IE and hepatitis C virus infection.12 IE manifestation has the potential to mimic vasculitis although mechanisms behind infection-induced PR3-ANCA are not well understood. It could be that an infectious process induces production of ANCA through non-specific B cell activation or autoimmunisation after the release of PR3 from neutrophils.6 13
To our knowledge, initial presentation with both spondylodiscitis and ANCA-positive vascilitis has not been described before.
Learning points.
-
▶
Vasculitic rash and positive cANCA do not always imply vasculitis alone. Chronic infection may mimic vasculitis.
-
▶
Blood cultures and TTE are non-invasive and relatively inexpensive investigations, and should be considered in the first instance in patients presenting with pyrexia of unclear origin. TOE may be needed to guide surgical planning. It should also be considered when there remains a high index of clinical suspicion despite a negative transthoracic study.
-
▶
Abdominal malignancy should be carefully excluded in right-sided endocarditis, if the culture-positive organism is potentially of bowel origin and there is no clear origin of endogenous bacteraemia.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med 2001;345:1318–30 [DOI] [PubMed] [Google Scholar]
- 2.Chan P, Ogilby JD, Segal B. Tricuspid valve endocarditis. Am Heart J 1989;117:1140–6 [DOI] [PubMed] [Google Scholar]
- 3.Miro JM, del Rio A, Mestres CA. Infective endocarditis in intravenous drug abusers and HIV-1 infected patients. Infect Dis Clin North Am 2002;16:273–95 [DOI] [PubMed] [Google Scholar]
- 4.Sandre RM, Shafran SD. Infective endocarditis: review of 135 cases over 9 years. Clin Infect Dis 1996;22:276–86 [DOI] [PubMed] [Google Scholar]
- 5.Heiro M, Helenizes H, Makila S, et al. Infective endocarditis in a Finnish teaching hospital: a study on 326 episodes treated during 1980-2004. Heart 2006;92:1457–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nandakumar R, Raju G. Isolated tricuspid valve endocarditis in nonaddicted patients: a diagnostic challenge. Am J Med Sci 1997;314:207–12 [DOI] [PubMed] [Google Scholar]
- 7.Evangelista A, Gonzalez-Alujas MT. Echocardiography in infective endocarditis. Heart 2004;90:614–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prendergast BD. The changing face of infective endocarditis. Heart 2006;92:879–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulleman D, Phillipe P, Senneville E. Streptococcal and enterococcal spondylodiscitis (vertebral osteomyelitis). High incidence of infective endocarditis in 50 cases. J Rhematol 2006;33:91–7 [PubMed] [Google Scholar]
- 10.Choi HK, Lamprecht P, Niles JL, et al. Subacute bacterial endocarditis with positive cytoplasmic antineutrophile cytoplasma antibodies and antiproteinase 3 antibodies. Arthritis Rheum 2000;43:226–31 [DOI] [PubMed] [Google Scholar]
- 11.Bauer A, Jabs WJ, Sufke S, et al. Vasculitic purpura with antineutrophil cytoplasmic antibody-positive acute renal failure in a patient with Streptococcus bovis case and Neisseria subflava bacterieamia and subacute endocarditis. Clin Nephrol 2004. 62:144–8 [DOI] [PubMed] [Google Scholar]
- 12.Bonaci-Nikolic B, Andrejevic S, Pavlovic M, et al. Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: diagnostic and therapeutic challenge. Clin Rheumatol 2010;29:893–904 [DOI] [PubMed] [Google Scholar]
- 13.Csernok E, Lamprecht P, Gross WL. Clinical and immunological features of drug induced and infection-induced proteinase 3-antineutrophil cytoplasmic antibodies and myeloperoxidase-antineutrophil cytoplasmic antibodies and vasculitis. Curr Opin Rheumatol 2010. ;22:43–8 [DOI] [PubMed] [Google Scholar]


