Abstract
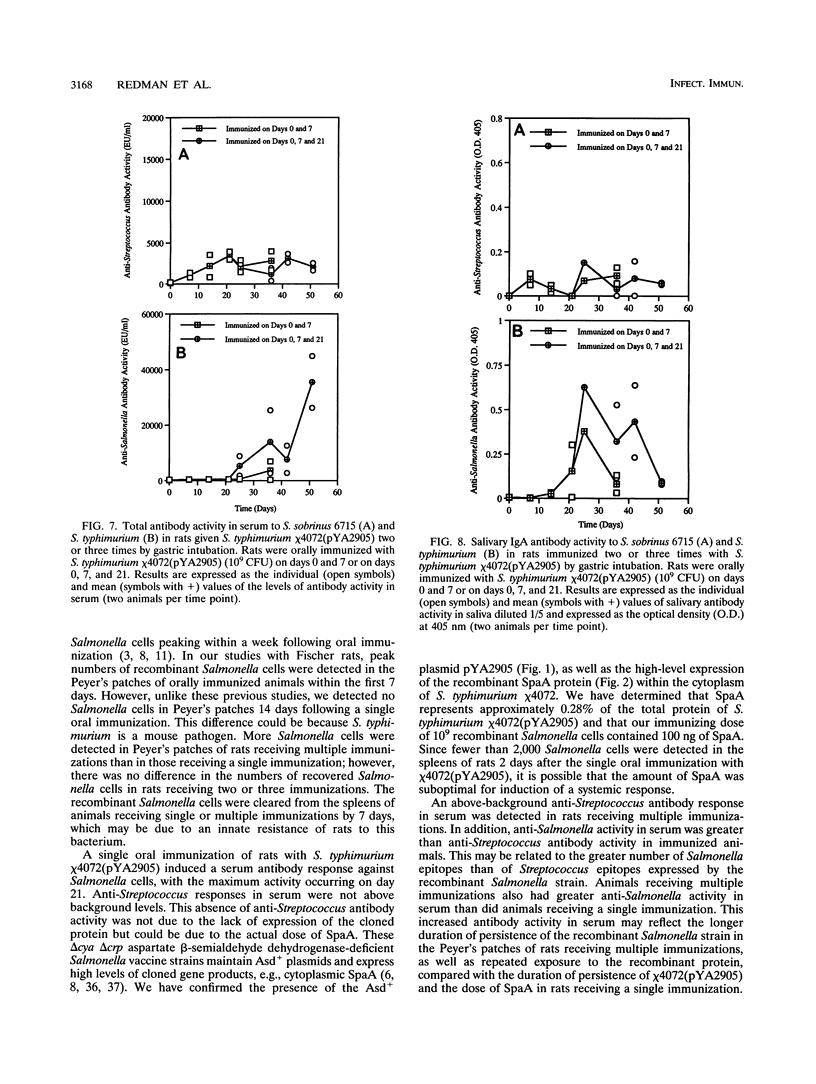
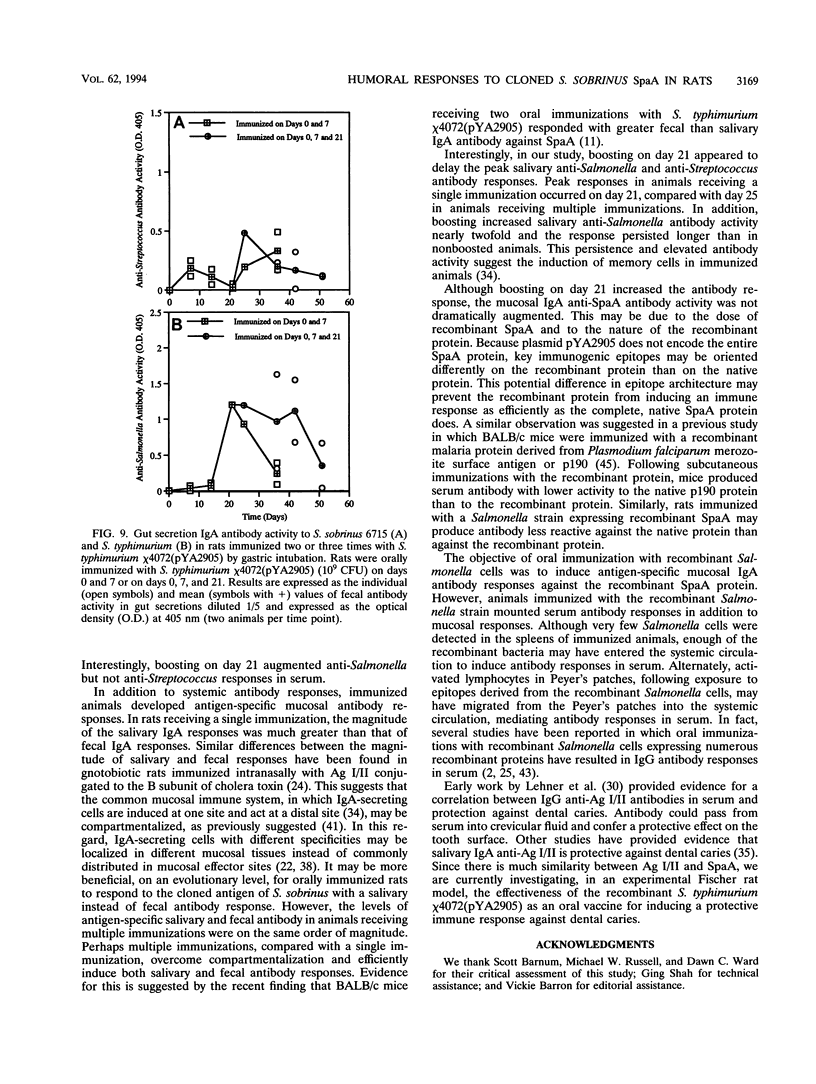
Recombinant Salmonella typhimurium has been used as an oral vaccine for various microbial pathogens. Here we report immune responses in Fischer rats orally immunized with a recombinant S. typhimurium strain encoding surface protein antigen A (SpaA) of Streptococcus sobrinus. The attenuated S. typhimurium chi 4072 delta cya delta crp delta asd mutant used in this study contains the Asd+ plasmid pYA2905 expressing a fragment of the SpaA protein. Salmonella cells were cleared from spleens by 7 days and from Peyer's patches by 14 days in rats receiving a single oral immunization of 10(9) CFU of chi 4072. In animals receiving multiple (i.e., days 0 and 7 or days 0, 7, and 21) immunizations, Salmonella cells were cleared from the Peyer's patches by 25 days following the initial immunization. Antigen-specific systemic and mucosal antibody responses were greater in rats receiving multiple immunizations than in those receiving a single immunization. Serum anti-Salmonella activity was potentiated following boosting on day 21. Mucosal immunoglobulin A antibody responses were also greater in rats receiving multiple immunizations than in rats receiving a single immunization. Anti-Salmonella and anti-Streptococcus immunoglobulin A activity persisted longer in rats boosted on day 21 than in rats immunized on days 0 and 7. These data indicate that oral immunization of rats with the recombinant S. typhimurium chi 4072(pYA2905) vaccine induces systemic as well as mucosal antibody responses specific to the Salmonella cells and to the cloned SpaA protein. This is the first report of the use of an attenuated mutant of the murine pathogen S. typhimurium as an oral vaccine in rats.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleiweis A. S., Oyston P. C., Brady L. J. Molecular, immunological and functional characterization of the major surface adhesin of Streptococcus mutans. Adv Exp Med Biol. 1992;327:229–241. doi: 10.1007/978-1-4615-3410-5_25. [DOI] [PubMed] [Google Scholar]
- Brown A., Hormaeche C. E., Demarco de Hormaeche R., Winther M., Dougan G., Maskell D. J., Stocker B. A. An attenuated aroA Salmonella typhimurium vaccine elicits humoral and cellular immunity to cloned beta-galactosidase in mice. J Infect Dis. 1987 Jan;155(1):86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- Cao Y., Wen Z., Lu D. Construction of a recombinant oral vaccine against Salmonella typhi and Salmonella typhimurium. Infect Immun. 1992 Jul;60(7):2823–2827. doi: 10.1128/iai.60.7.2823-2827.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Goldschmidt R. M., Fletchall N. B., Kelly S. M. Avirulent Salmonella typhimurium delta cya delta crp oral vaccine strains expressing a streptococcal colonization and virulence antigen. Vaccine. 1988 Apr;6(2):155–160. doi: 10.1016/s0264-410x(88)80020-3. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Nakayama K., Kelly S. M. Recombinant avirulent Salmonella vaccine strains with stable maintenance and high level expression of cloned genes in vivo. Immunol Invest. 1989 Jan-May;18(1-4):583–596. doi: 10.3109/08820138909112265. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Russell M. W., Lycke N., Lindblad M., Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989 Apr;57(4):1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett T. A., Jagusztyn-Krynicka E. K., Curtiss R., 3rd Immune responses to Streptococcus sobrinus surface protein antigen A expressed by recombinant Salmonella typhimurium. Infect Immun. 1993 May;61(5):1859–1866. doi: 10.1128/iai.61.5.1859-1866.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Ealding W., Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J Immunol Methods. 1984 Feb 24;67(1):101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Manjula B. N., Scott J. R. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification, and comparison with streptococcal-derived M protein. J Exp Med. 1984 Apr 1;159(4):1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Nakayama K., Curtiss R., 3rd Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene. 1990 Sep 28;94(1):29–35. doi: 10.1016/0378-1119(90)90464-3. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Adherent interactions which may affect microbial ecology in the mouth. J Dent Res. 1984 Mar;63(3):378–385. doi: 10.1177/00220345840630030401. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R. M., Curtiss R., 3rd Cross-reactivity between the immunodominant determinant of the antigen I component of Streptococcus sobrinus SpaA protein and surface antigens from other members of the Streptococcus mutans group. Infect Immun. 1990 Jul;58(7):2276–2282. doi: 10.1128/iai.58.7.2276-2282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt R. M., Thoren-Gordon M., Curtiss R., 3rd Regions of the Streptococcus sobrinus spaA gene encoding major determinants of antigen I. J Bacteriol. 1990 Jul;172(7):3988–4001. doi: 10.1128/jb.172.7.3988-4001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G., Nikolova E., Russell M. W. Inhibition of Streptococcus mutans adherence to saliva-coated hydroxyapatite by human secretory immunoglobulin A (S-IgA) antibodies to cell surface protein antigen I/II: reversal by IgA1 protease cleavage. Infect Immun. 1992 Dec;60(12):5057–5064. doi: 10.1128/iai.60.12.5057-5064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson G. D., Hansen P. G., Underdown B. J. Further evidence that hepatic sources confer biliary antibody in the rat. Immunology. 1992 Jul;76(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Katz J., Harmon C. C., Buckner G. P., Richardson G. J., Russell M. W., Michalek S. M. Protective salivary immunoglobulin A responses against Streptococcus mutans infection after intranasal immunization with S. mutans antigen I/II coupled to the B subunit of cholera toxin. Infect Immun. 1993 May;61(5):1964–1971. doi: 10.1128/iai.61.5.1964-1971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Michalek S. M., Curtiss R., 3rd, Harmon C., Richardson G., Mestecky J. Novel oral vaccines: the effectiveness of cloned gene products on inducing secretory immune responses. Adv Exp Med Biol. 1987;216B:1741–1747. [PubMed] [Google Scholar]
- Kiyono H., Babb J. L., Michalek S. M., McGhee J. R. Cellular basis for elevated IgA responses in C3H/HeJ mice. J Immunol. 1980 Aug;125(2):732–737. [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Neutra M. R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992 Oct;72(4):853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med. 1993;4(2):159–176. doi: 10.1177/10454411930040020201. [DOI] [PubMed] [Google Scholar]
- LaPolla R. J., Haron J. A., Kelly C. G., Taylor W. R., Bohart C., Hendricks M., Pyati J. P., Graff R. T., Ma J. K., Lehner T. Sequence and structural analysis of surface protein antigen I/II (SpaA) of Streptococcus sobrinus. Infect Immun. 1991 Aug;59(8):2677–2685. doi: 10.1128/iai.59.8.2677-2685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Russell M. W., Caldwell J., Smith R. Immunization with purified protein antigens from Streptococcus mutans against dental caries in rhesus monkeys. Infect Immun. 1981 Nov;34(2):407–415. doi: 10.1128/iai.34.2.407-415.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J. In defense of mucosal surfaces. Development of novel vaccines for IgA responses protective at the portals of entry of microbial pathogens. Infect Dis Clin North Am. 1990 Jun;4(2):315–341. [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., Childers N. K., Katz J., Dertzbaugh M., Zhang S., Russell M. W., Macrina F. L., Jackson S., Mestecky J. Liposomes and conjugate vaccines for antigen delivery and induction of mucosal immune responses. Adv Exp Med Biol. 1992;327:191–198. doi: 10.1007/978-1-4615-3410-5_21. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr Determinants of the localization, magnitude, and duration of a specific mucosal IgA plasma cell response in enterically immunized rats. J Immunol. 1982 Mar;128(3):1311–1315. [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W. Immunization against dental caries. Curr Opin Dent. 1992 Sep;2:72–80. [PubMed] [Google Scholar]
- Russell M. W., Wu H. Y. Distribution, persistence, and recall of serum and salivary antibody responses to peroral immunization with protein antigen I/II of Streptococcus mutans coupled to the cholera toxin B subunit. Infect Immun. 1991 Nov;59(11):4061–4070. doi: 10.1128/iai.59.11.4061-4070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstedt A., Sandström G., Tärnvik A. Humoral and cell-mediated immunity in mice to a 17-kilodalton lipoprotein of Francisella tularensis expressed by Salmonella typhimurium. Infect Immun. 1992 Jul;60(7):2855–2862. doi: 10.1128/iai.60.7.2855-2862.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel T. J., Mayfield J. E., Tabatabai L. B., Wannemuehler M. J. Swine immunity to an attenuated Salmonella typhimurium mutant containing a recombinant plasmid which codes for production of a 31-kilodalton protein of Brucella abortus. Infect Immun. 1991 Sep;59(9):2941–2947. doi: 10.1128/iai.59.9.2941-2947.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süss G., Pink J. R. A recombinant malaria protein that can induce Th1 and CD8+ T cell responses without antibody formation. J Immunol. 1992 Aug 15;149(4):1334–1339. [PubMed] [Google Scholar]
- Takahashi I., Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Homology between surface protein antigen genes of Streptococcus sobrinus and Streptococcus mutans. FEBS Lett. 1989 Jun 5;249(2):383–388. doi: 10.1016/0014-5793(89)80664-7. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Russell M. W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993 Jan;61(1):314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]