Abstract
Intravitreal bevacizumab (Avastin) has been used off label for various conditions, including proliferative sickle cell retinopathy (PSR). Some authors have reported a more rapid resolution of the vitreous haemorrhage in Goldberg stage 4 PSR. Following injection of 1.25 mg of bevacizumab in each eye in a case of PSR (right eye with stage 3 sea fan neovascularisation and left eye with organising vitreous haemorrhage), we recently found a surprising association with significant secondary hyphaema on the fifth day post injection in the eye with vitreous haemorrhage. After 4 weeks, there was a dramatic fibrotic resolution of the stage 3 sea fan in the other eye. Systemic bevacizumab as used in the management of colorectal cancer has been associated with bleeding diathesis in some cases, as well as with deep venous thrombosis. The rheological profile of bevacizumab is not clear-cut at the moment and the drug should therefore be used with caution in cases of stage 4 PSR.
Background
We are only just beginning to gain experience with the use intravitreal bevacizumab (Avastin) for various indications,1–3 one of which is proliferative sickle cell retinopathy (PSR). Our findings presented in this report seemingly contradict the report of Shaikh3 who reported a quicker resolution of vitreous haemorrhage following this injection. At present, the vitreous haemorrhage in our patient has still not improved.
Case presentation
A 25-year-old male patient with sickle cell (SC) genotype presented with a 3 month history of progressive loss of vision in the left eye. The visual acuity on presentation was 6/5 right eye and counting fingers on the temporal field, left eye. In the right eye he had sea fans, black sunburst lesions, schisis cavities, and salmon patch haemorrhages. In the left eye he had relatively organised vitreous haemorrhage with poor visualisation of details. However, with indirect ophthalmoscopy it was possible to discern areas of haemorrhage spanning 360° associated with neovascularisation, some black sunburst and schisis cavities.
The patient was counselled on the nature of the problem and advised that a fluorescein angiogram would be necessary to confirm the extent of the lesions. Treatment options of laser, cryotherapy and intracameral bevacizumab were discussed. The patient needed to be appraised of the off licence nature of bevacizumab. Because of the dense haemorrhage in the left eye, it was decided that bevacizumab would be administered following which laser therapy may be applied only to the right eye.
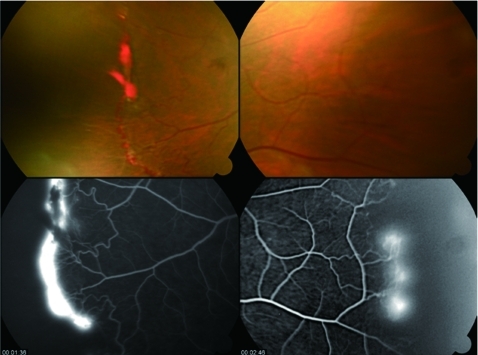
The fluorescein angiogram confirmed leaking areas of sea fan neovascularisation in the right eye (fig 1), both nasally and temporally, with distal avascularised zones. It was not possible to get clear pictures in the left eye due to the haemorrhage.
Figure 1.
Right eye before the injection of bevacizumab. Temporal sea fan neovascularisation with areas of haemorrhage, and nasal neovascular tufts on arteriovenous anastomosis (top) with corresponding areas of leakage of fluorescein (below).
Bevacizumab, 1.25 mg, was administered into both eyes by atraumatic trans-scleral pars plana injection 3.5 mm temporally behind the limbus. The following day, the eyes were inspected. There was no fresh haemorrhage in either eye, either anteriorly or posteriorly. The old vitreous haemorrhage in the left eye remained unchanged.
On day 5, a crescentic hyphaema was noticed in the left eye while there was no change in the sea fan formations in the right eye. Slit lamp examination of the left eye showed that the anterior chamber was densely packed with blood cells, contiguous with the cells in the vitreous cavity. The intraocular pressure was 12 mm Hg in both eyes.
Bed rest and double padding of the eyes were ordered. This did not result in any significant change. By day 12 there was about a 30% hyphaema, with red cells in the anterior segment. A paracentesis was carried out the following day with no recurrence of hyphaema. However, the vitreous haemorrhage remained unchanged.
By the 26th day following bevacizumab injection, an examination of the right eye showed a fibrotic regression of the sea fan and haemorrhage. There was still no change in the vitreous haemorrhage in the left eye.
A repeat fluorescein angiogram was carried out on the 27th day after bevacizumab injection.
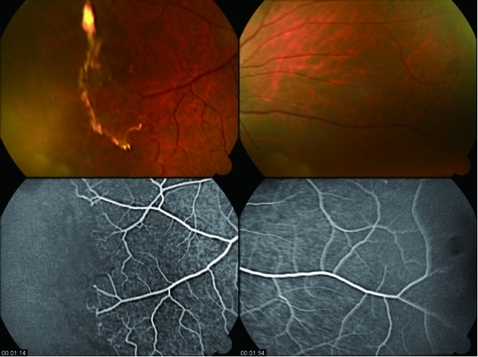
This confirmed that there was no more leakage of the sea fan neovascular lesions, both temporally and nasally (fig 2). It was considered unnecessary to apply laser therapy to the avascular zone of the eye.
Figure 2.
Same right eye 3 weeks after injection of bevacizumab. Temporal sea fan and haemorrhage has resolved into a white fibrotic strand, and there is an absence of leakage both nasally and temporally.
Outcome and follow-up
There was a resolution of the stage 3 sea fan in the right eye, while there was a secondary hyphaema (by day 5) with no resolution of the vitreous haemorrhage in the left eye. A paracentesis of the hyphaema, however, did not lead to a rebleed.
Discussion
Bevacizumab has been used off licence (US Food and Drug Administration) for about 3–4 years for the management of eye disorders. As is well known, it was originally developed for use in the management of metastatic colorectal cancer.4,5
A proper evaluation of its long term effects is therefore not possible at present. A number of authors have reported a favourable outcome concerning its use in sickle cell retinopathy.3,6 Adverse events with intra-cameral bevacizumab have not been commonly reported, however.
This particular case demonstrates a clear beneficial effect in the eye, with the nascent sea fan lesions in the right eye. How long this effect would be maintained is not clear. This is because the original incentive for neovascularisation, which is capillary closure and peripheral retinal hypoxia, has probably not been redressed. It is therefore plausible that laser therapy may still be indicated.
The occurrence of hyphaema following the injection in the fellow eye with the vitreous haemorrhage is a little more puzzling. It may be surmised that further bleeding may have triggered a spillover of blood into the anterior segment. Because the hyphaema was not noticed until the fifth day after the injection, it seems unlikely that it was iatrogenically induced following the mechanical introduction of the needle, which was a 27 gauge insulin injection type needle. Also, the increase in the degree of the hyphaema following the introduction of conservative measures (bed rest and double padding) suggests that there was an ongoing process.
Spontaneous hyphaema has been associated with sickle cell disease,7 although the exact pathogenesis is unknown. It is not implausible that vitreous haemorrhage could spill over into the anterior chamber causing hyphaema. With reference to this particular case, the anterior chamber was filled with blood cells which appeared to be a spillover of the vitreous haemorrhage, akin to the spillover of silicone from the posterior segment.7
It is not clear how or if the bevacizumab injection increased the likelihood of the hyphaema, but certainly this is a complication that should be borne in mind if bevacizumab is to be injected into vitreous haemorrhage associated with SC retinopathy. This finding is antithetical to that of Shaikh3 who reported that vitreous haemorrhage associated with SC cleared more readily after bevacizumab was injected. So far, in our patient there has been no resolution of the vitreous haemorrhage.
Severe bleeding has been reported by the manufacturers, Genentech, as a side effect of bevacizumab (http://www.avastin.com/avastin/index). The drug information document states:
“Treatment with Avastin can result in serious bleeding, including coughing up blood, bleeding in the stomach, vomiting of blood, bleeding in the brain, nosebleeds, and vaginal bleeding. These events occurred up to 5 times more often in people who received Avastin. Across cancer types, 1.2% to 4.6% of people who received Avastin experienced severe to fatal bleeding. People who have recently coughed up blood (greater than or equal to a half teaspoon of red blood) or have serious bleeding should not receive Avastin. Treatment with Avastin should be permanently stopped if serious bleeding occurs.”
This suggests that bevacizumab has some rheological effect tending towards a propensity to encourage bleeding. Other literature, on the other hand, ironically points to bevacizumab as encouraging clot formation (http://www.defendingthetruth.com/health-care/21979-genentechs-avastin-linked-vein-clotting-risk-analysis.html). These seemingly contradictory rheological properties require further investigation and clarification.
In conclusion, care must be taken in the use of intracameral bevacizumab for the management of sickle cell retinopathy associated with vitreous haemorrhage.
Learning points
Intracameral bevacizumab is apparently useful in the management of stage 3 proliferative sickle cell retinopathy, leading to a fibrotic resolution of the sea fan.
It may ironically lead to a secondary hyphaema if there is already a vitreous haemorrhage.
That it leads to a resolution of proliferative sickle cell retinopathy related vitreous haemorrhage as reported elsewhere is contradicted by our experience.
Acknowledgments
I thank the nurses who worked with me on this case.
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Grisanti S, Ziemssen F: Bevacizumab: off-label use in ophthalmology. Indian J Ophthalmol 2007; 55: 417–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunther JB, Alaweel MM. Bevacizumab (Avastin) for the treatment of ocular disease. Survey of Ophthalmology 2009; 54: 372–400 [DOI] [PubMed] [Google Scholar]
- 3.Shaikh S. Intravitreal bevacizumab (Avastin) for the treatment of proliferative sickle retinopathy. Indian J Ophthalmol 2008; 56: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim R. Introduction, mechanism of action and rationale for anti-vascular endothelial growth factor drugs in age-related macular degeneration. Indian J Ophthalmol 2007; 55: 413–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal NH, Saltz LB. Evolving treatment of advanced colon cancer. Annu Rev Med 2009; 60: 207–19 [DOI] [PubMed] [Google Scholar]
- 6.Siqueira RC, Costa RA, Scott IU, et al. Intravitreal bevacizumab (Avastin) injection associated with regression of retinal neovascularization caused by sickle cell retinopathy. Acta Ophthalmol Scand 2006; 84: 834–5 [DOI] [PubMed] [Google Scholar]
- 7.Murdoch IA, Dos Anjos R, Parsons JM, et al. Spontaneous hyphaema in childhood. Eur J Pediatr 1991; 150: 717–8 [DOI] [PubMed] [Google Scholar]