Abstract
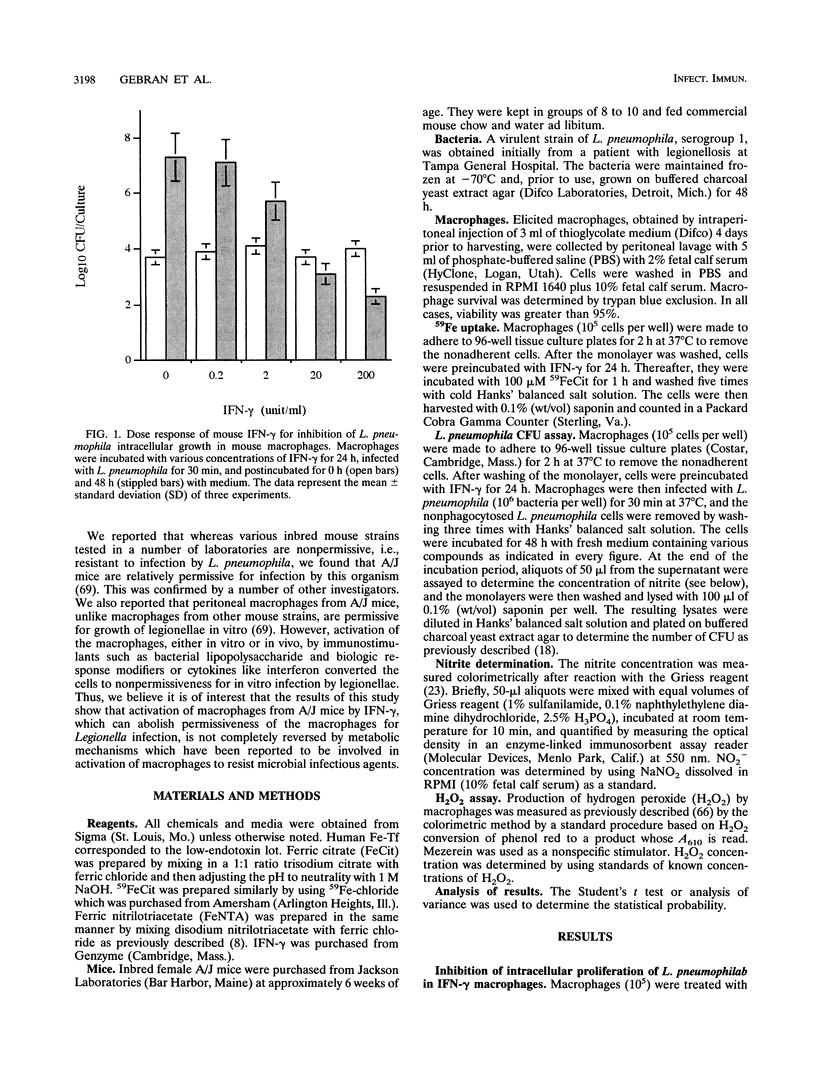
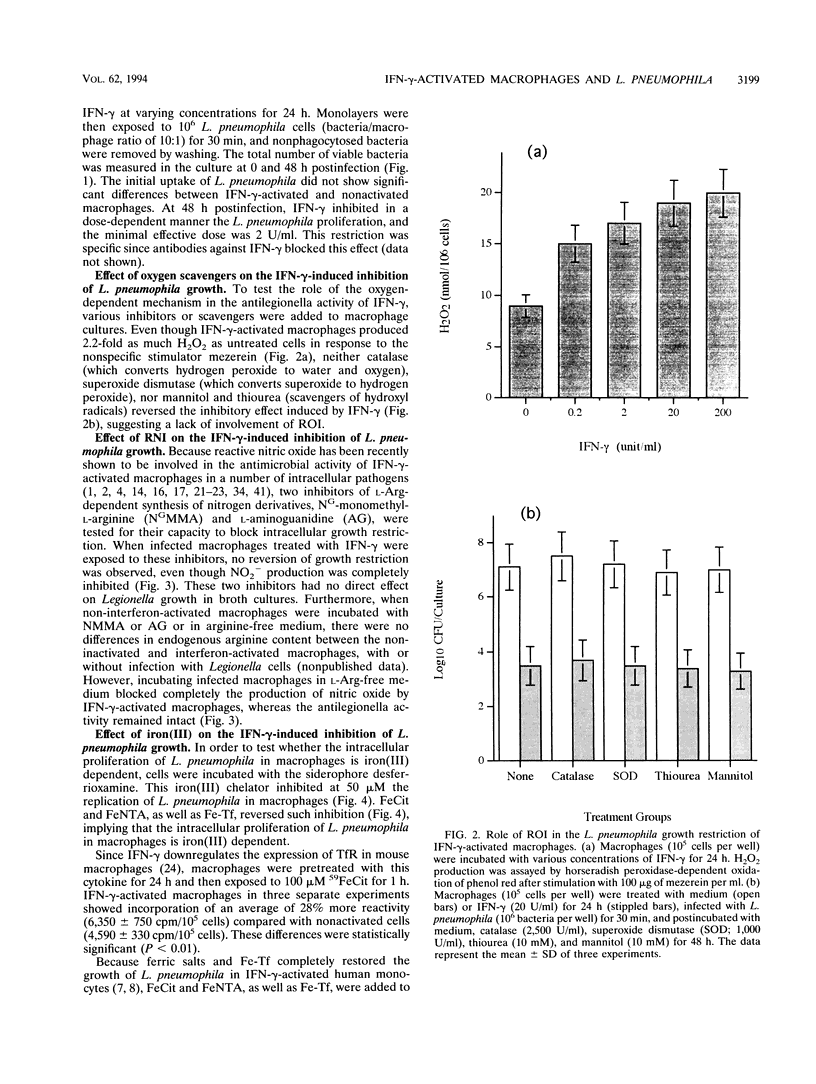
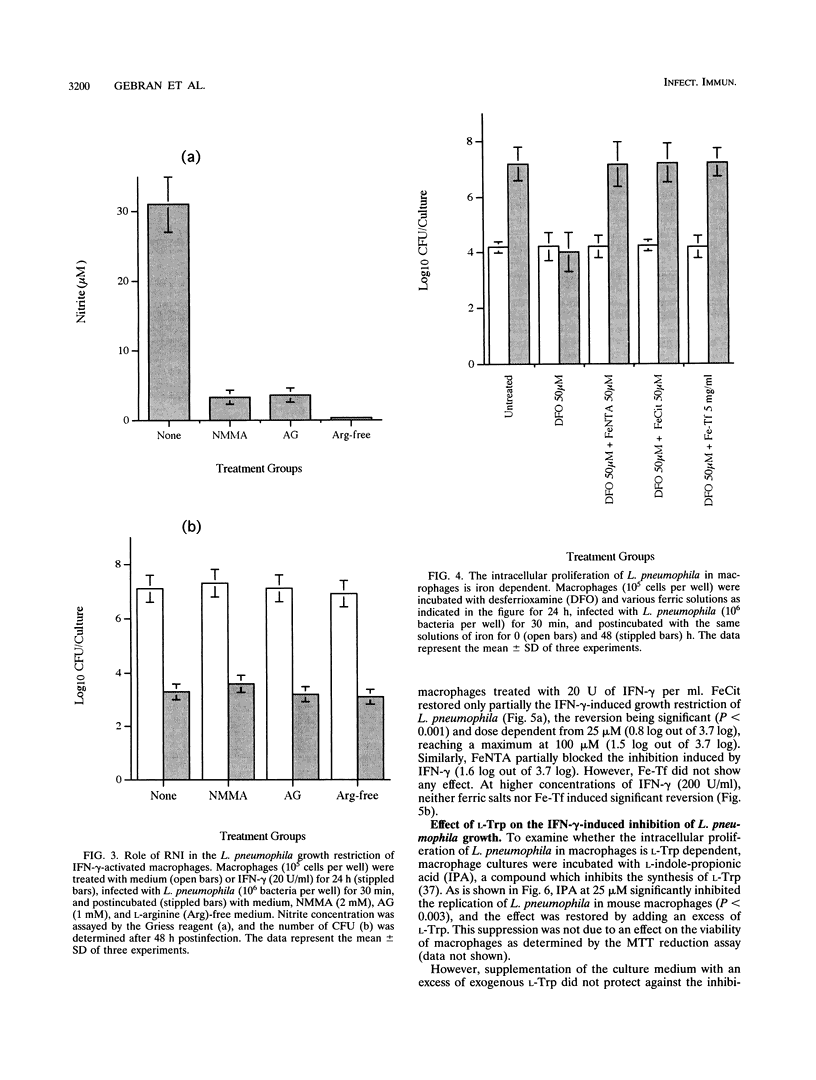
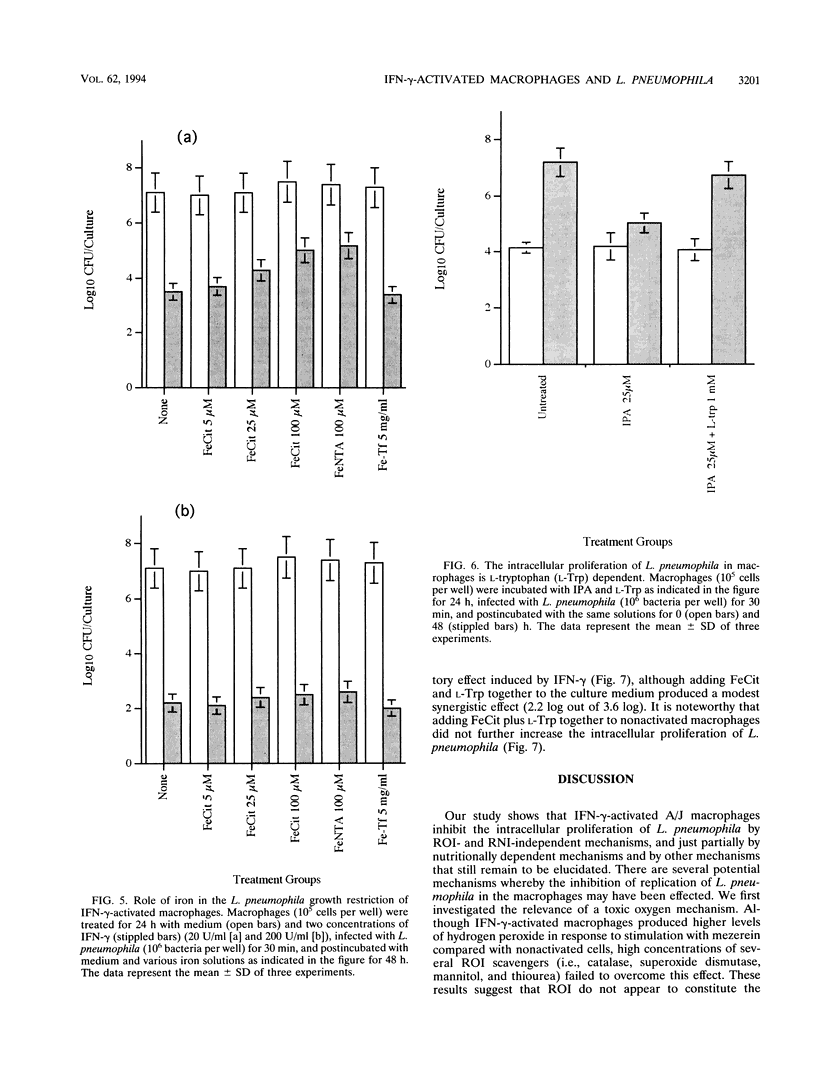
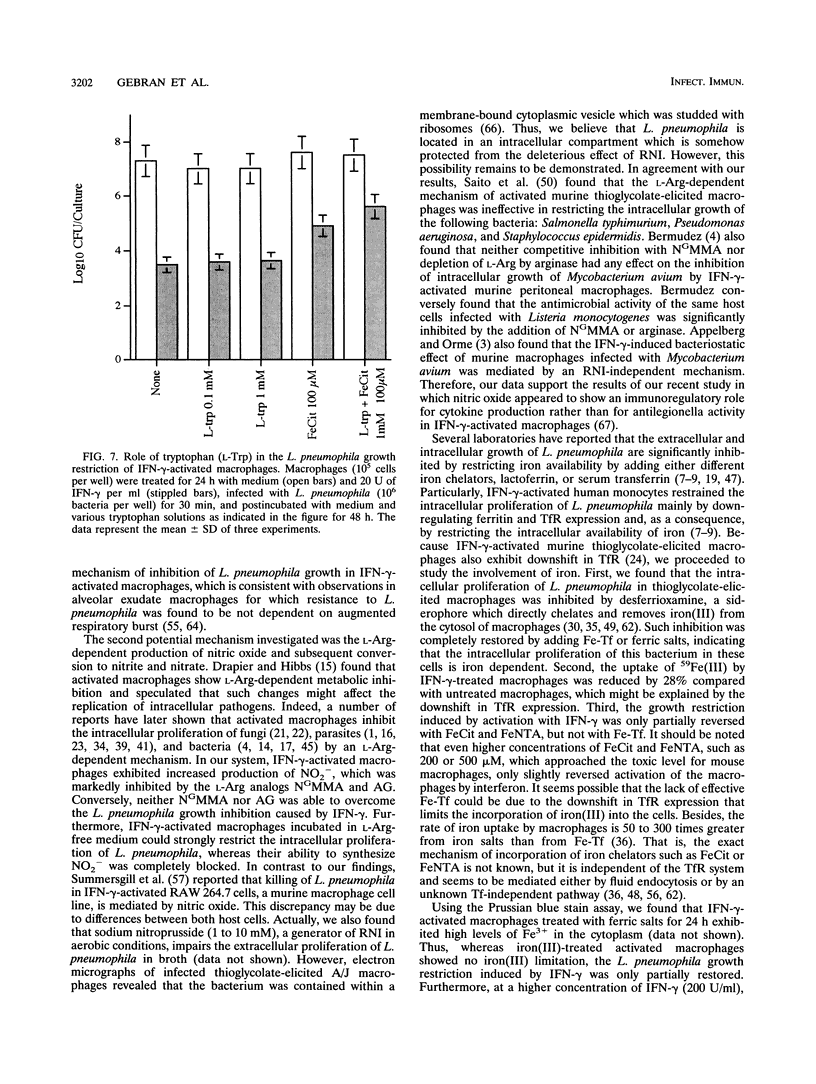
A/J mouse macrophages infected with Legionella pneumophila and treated with gamma interferon (IFN-gamma) in vitro developed potent antimicrobial activity. This antilegionella activity was independent of the macrophage capacity to generate reactive oxygen intermediates, since the oxygen radical scavengers catalase, superoxide dismutase, mannitol, and thiourea had no effect on the antilegionella activity of IFN-gamma-activated macrophages. Likewise, whereas the ability of IFN-gamma-activated macrophages to synthesize reactive nitrogen intermediates was markedly inhibited by the L-arginine (Arg) analogs, NG-monomethyl-L-arginine and L-aminoguanidine, as well as by incubation in L-Arg-free medium, their ability to inhibit the intracellular growth of L. pneumophila remained intact. The intracellular growth of L. pneumophila in A/J macrophages was inhibited by the iron(III) chelator desferrioxamine and reversed by Fe-transferrin as well as by ferric salts. Additionally, IFN-gamma-activated macrophages incorporated 28% less 59Fe(III) compared with nonactivated cells. Nonetheless, only partial blocking of growth restriction was observed when IFN-gamma-stimulated macrophages were saturated with iron(III). Indole-propionic acid, which appears to inhibit the biosynthesis of L-tryptophan (L-Trp), was an L-Trp-reversible growth inhibitor of L. pneumophila in macrophages, implying that the intracellular replication of this pathogen is also L-Trp dependent. However, an excess of exogenous L-Trp did not reverse the growth inhibition due to IFN-gamma, though a small synergistic effect was observed when the culture medium was supplemented with both iron(III) and L-Trp. We conclude that IFN-gamma-activated macrophages inhibit the intracellular proliferation of L. pneumophila by reactive oxygen intermediate- and reactive nitrogen intermediate-independent mechanisms and just partially by nutritionally dependent mechanisms. We also suggest that additional mechanisms, still unclear, may be involved, since complete reversion was never obtained and since at higher concentrations of IFN-gamma, iron(III) did not induce any significant reversion in the L. pneumophila growth inhibition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Anthony L. S., Morrissey P. J., Nano F. E. Growth inhibition of Francisella tularensis live vaccine strain by IFN-gamma-activated macrophages is mediated by reactive nitrogen intermediates derived from L-arginine metabolism. J Immunol. 1992 Mar 15;148(6):1829–1834. [PubMed] [Google Scholar]
- Appelberg R., Orme I. M. Effector mechanisms involved in cytokine-mediated bacteriostasis of Mycobacterium avium infections in murine macrophages. Immunology. 1993 Nov;80(3):352–359. [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages. The role of nitric oxide. Clin Exp Immunol. 1993 Feb;91(2):277–281. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N., Nash T. W., Horwitz M. A. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986 Oct 15;137(8):2662–2669. [PubMed] [Google Scholar]
- Bianchi M., Bertini R., Ghezzi P. Induction of indoleamine dioxygenase by interferon in mice: a study with different recombinant interferons and various cytokines. Biochem Biophys Res Commun. 1988 Apr 15;152(1):237–242. doi: 10.1016/s0006-291x(88)80705-8. [DOI] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989 May;83(5):1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Lactoferrin inhibits or promotes Legionella pneumophila intracellular multiplication in nonactivated and interferon gamma-activated human monocytes depending upon its degree of iron saturation. Iron-lactoferrin and nonphysiologic iron chelates reverse monocyte activation against Legionella pneumophila. J Clin Invest. 1991 Oct;88(4):1103–1112. doi: 10.1172/JCI115409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Regulation of transferrin receptor expression and ferritin content in human mononuclear phagocytes. Coordinate upregulation by iron transferrin and downregulation by interferon gamma. J Clin Invest. 1993 Mar;91(3):969–976. doi: 10.1172/JCI116318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Lehmann L. K., Landry G. J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986 Aug;53(2):347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin J. M., Borden E. C., Byrne G. I. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J Interferon Res. 1989 Jun;9(3):329–337. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- Carlin J. M., Borden E. C., Sondel P. M., Byrne G. I. Biologic-response-modifier-induced indoleamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J Immunol. 1987 Oct 1;139(7):2414–2418. [PubMed] [Google Scholar]
- Carlin J. M., Borden E. C., Sondel P. M., Byrne G. I. Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol. 1989 Jan;45(1):29–34. doi: 10.1002/jlb.45.1.29. [DOI] [PubMed] [Google Scholar]
- Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991 Jan;132(1):150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Fischer-Stenger K., Marciano-Cabral F. The arginine-dependent cytolytic mechanism plays a role in destruction of Naegleria fowleri amoebae by activated macrophages. Infect Immun. 1992 Dec;60(12):5126–5131. doi: 10.1128/iai.60.12.5126-5131.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991 Sep;59(9):3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebran S. J., Newton C. A., Yamamoto Y., Klein T. W., Friedman H. A rapid colorimetric assay for evaluating Legionella pneumophila growth in macrophages in vitro. J Clin Microbiol. 1994 Jan;32(1):127–130. doi: 10.1128/jcm.32.1.127-130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebran S. J., Newton C., Yamamoto Y., Widen R., Klein T. W., Friedman H. Macrophage permissiveness for Legionella pneumophila growth modulated by iron. Infect Immun. 1994 Feb;62(2):564–568. doi: 10.1128/iai.62.2.564-568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. R., Pine L., Reeves M. W., Harrell W. K. Amino acid requirements of Legionella pneumophila. J Clin Microbiol. 1980 Mar;11(3):286–291. doi: 10.1128/jcm.11.3.286-291.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Hamilton T. A., Gray P. W., Adams D. O. Expression of the transferrin receptor on murine peritoneal macrophages is modulated by in vitro treatment with interferon gamma. Cell Immunol. 1984 Dec;89(2):478–488. doi: 10.1016/0008-8749(84)90348-4. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen W. A., Rose R. M., Wasserman A. S., Kalb T. H., Anton K., Remold H. G. In vitro activation of the antibacterial activity of human pulmonary macrophages by recombinant gamma interferon. J Infect Dis. 1987 Mar;155(3):574–577. doi: 10.1093/infdis/155.3.574. [DOI] [PubMed] [Google Scholar]
- Jiang X., Baldwin C. L. Iron augments macrophage-mediated killing of Brucella abortus alone and in conjunction with interferon-gamma. Cell Immunol. 1993 May;148(2):397–407. doi: 10.1006/cimm.1993.1121. [DOI] [PubMed] [Google Scholar]
- Kleber E. E., Torrance J. D., Bothwell T. H., Simon M. O., Charlton R. W. Mobilisation of iron from peritoneal rat macrophages by desferrioxamine. Scand J Haematol. 1981 Sep;27(3):209–218. doi: 10.1111/j.1600-0609.1981.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Klein T. W., Yamamoto Y., Brown H. K., Friedman H. Interferon-gamma induced resistance to Legionella pneumophila in susceptible A/J mouse macrophages. J Leukoc Biol. 1991 Jan;49(1):98–103. doi: 10.1002/jlb.49.1.98. [DOI] [PubMed] [Google Scholar]
- Lane T. E., Wu-Hsieh B. A., Howard D. H. Gamma interferon cooperates with lipopolysaccharide to activate mouse splenic macrophages to an antihistoplasma state. Infect Immun. 1993 Apr;61(4):1468–1473. doi: 10.1128/iai.61.4.1468-1473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. E., Wu-Hsieh B. A., Howard D. H. Iron limitation and the gamma interferon-mediated antihistoplasma state of murine macrophages. Infect Immun. 1991 Jul;59(7):2274–2278. doi: 10.1128/iai.59.7.2274-2278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Lipschitz D. A., Dugard J., Simon M. O., Bothwell T. H., Charlton R. W. The site of action of desferrioxamine. Br J Haematol. 1971 Apr;20(4):395–404. doi: 10.1111/j.1365-2141.1971.tb07051.x. [DOI] [PubMed] [Google Scholar]
- MacDonald R. A., MacSween R. N., Pechet G. S. Iron metabolism by reticuloendothelial cells in vitro. Physical and chemical conditions, lipotrope deficiency, and acute inflammation. Lab Invest. 1969 Sep;21(3):236–245. [PubMed] [Google Scholar]
- Mandelbaum-Shavit F., Barak V., Saheb-Tamimi K., Grossowicz N. Susceptibility of Legionella pneumophila grown extracellularly and in human monocytes to indole-3-propionic acid. Antimicrob Agents Chemother. 1991 Dec;35(12):2526–2530. doi: 10.1128/aac.35.12.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor E., Sarov I. Inhibition of Rickettsia conorii growth by recombinant tumor necrosis factor alpha: enhancement of inhibition by gamma interferon. Infect Immun. 1990 Jun;58(6):1886–1890. doi: 10.1128/iai.58.6.1886-1890.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauël J., Ransijn A., Buchmüller-Rouiller Y. Killing of Leishmania parasites in activated murine macrophages is based on an L-arginine-dependent process that produces nitrogen derivatives. J Leukoc Biol. 1991 Jan;49(1):73–82. doi: 10.1002/jlb.49.1.73. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Granger A. M., Teitelbaum R. F. Gamma interferon-activated human macrophages and Toxoplasma gondii, Chlamydia psittaci, and Leishmania donovani: antimicrobial role of limiting intracellular iron. Infect Immun. 1991 Dec;59(12):4684–4686. doi: 10.1128/iai.59.12.4684-4686.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Szuro-Sudol A., Wellner D., Oca M. J., Granger A. M., Libby D. M., Rothermel C. D., Rubin B. Y. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989 Mar;57(3):845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Fernández M. A., Fernández M. A., Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol Lett. 1992 Jun;33(1):35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- Nash T. W., Libby D. M., Horwitz M. A. IFN-gamma-activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1988 Jun 1;140(11):3978–3981. [PubMed] [Google Scholar]
- Park J., Rikihisa Y. L-arginine-dependent killing of intracellular Ehrlichia risticii by macrophages treated with gamma interferon. Infect Immun. 1992 Sep;60(9):3504–3508. doi: 10.1128/iai.60.9.3504-3508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984 Feb;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D., Baker E. The uptake of inorganic iron complexes by human melanoma cells. Biochim Biophys Acta. 1991 Jun 7;1093(1):20–28. doi: 10.1016/0167-4889(91)90133-i. [DOI] [PubMed] [Google Scholar]
- Roberts S., Bomford A. Chelation of transferrin iron by desferrioxamine in K562 cells. The partition of iron between ferrioxamine and ferritin. Biochem J. 1988 Sep 15;254(3):869–875. doi: 10.1042/bj2540869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S., Onozuka K., Shinomiya H., Nakano M. Sensitivity of bacteria to NaNO2 and to L-arginine-dependent system in murine macrophages. Microbiol Immunol. 1991;35(4):325–329. doi: 10.1111/j.1348-0421.1991.tb01561.x. [DOI] [PubMed] [Google Scholar]
- Schmitz J. L., Carlin J. M., Borden E. C., Byrne G. I. Beta interferon inhibits Toxoplasma gondii growth in human monocyte-derived macrophages. Infect Immun. 1989 Oct;57(10):3254–3256. doi: 10.1128/iai.57.10.3254-3256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Ferreira A., Altszuler R., Nussenzweig V., Nussenzweig R. S. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987 Sep 15;139(6):2020–2025. [PubMed] [Google Scholar]
- Shemer-Avni Y., Wallach D., Sarov I. Reversion of the antichlamydial effect of tumor necrosis factor by tryptophan and antibodies to beta interferon. Infect Immun. 1989 Nov;57(11):3484–3490. doi: 10.1128/iai.57.11.3484-3490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerrett S. J., Martin T. R. Alveolar macrophage activation in experimental legionellosis. J Immunol. 1991 Jul 1;147(1):337–345. [PubMed] [Google Scholar]
- Sturrock A., Alexander J., Lamb J., Craven C. M., Kaplan J. Characterization of a transferrin-independent uptake system for iron in HeLa cells. J Biol Chem. 1990 Feb 25;265(6):3139–3145. [PubMed] [Google Scholar]
- Summersgill J. T., Powell L. A., Buster B. L., Miller R. D., Ramirez J. A. Killing of Legionella pneumophila by nitric oxide in gamma-interferon-activated macrophages. J Leukoc Biol. 1992 Dec;52(6):625–629. doi: 10.1002/jlb.52.6.625. [DOI] [PubMed] [Google Scholar]
- Tesh M. J., Miller R. D. Amino acid requirements for Legionella pneumophila growth. J Clin Microbiol. 1981 May;13(5):865–869. doi: 10.1128/jcm.13.5.865-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Gamma-interferon-induced inhibition of the growth of Rickettsia prowazekii in fibroblasts cannot be explained by the degradation of tryptophan or other amino acids. Infect Immun. 1986 Jul;53(1):38–46. doi: 10.1128/iai.53.1.38-46.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Shimamoto Y., Yoshida S., Suga K., Mizuguchi Y., Kohashi O., Yamaguchi M. Intracellular multiplication of Legionella pneumophila in HL-60 cells differentiated by 1,25-dihydroxyvitamin D3 and the effect of interferon gamma. J Leukoc Biol. 1993 Jul;54(1):40–46. doi: 10.1002/jlb.54.1.40. [DOI] [PubMed] [Google Scholar]
- Werner E. R., Bitterlich G., Fuchs D., Hausen A., Reibnegger G., Szabo G., Dierich M. P., Wachter H. Human macrophages degrade tryptophan upon induction by interferon-gamma. Life Sci. 1987 Jul 20;41(3):273–280. doi: 10.1016/0024-3205(87)90149-4. [DOI] [PubMed] [Google Scholar]
- White G. P., Jacobs A. Iron uptake by Chang cells from transferrin, nitriloacetate and citrate complexes: the effects of iron-loading and chelation with desferrioxamine. Biochim Biophys Acta. 1978 Oct 3;543(2):217–225. doi: 10.1016/0304-4165(78)90066-1. [DOI] [PubMed] [Google Scholar]
- Woodman J. P., Dimier I. H., Bout D. T. Human endothelial cells are activated by IFN-gamma to inhibit Toxoplasma gondii replication. Inhibition is due to a different mechanism from that existing in mouse macrophages and human fibroblasts. J Immunol. 1991 Sep 15;147(6):2019–2023. [PubMed] [Google Scholar]
- Wu-Hsieh B. A., Howard D. H. Intracellular growth inhibition of Histoplasma capsulatum induced in murine macrophages by recombinant gamma interferon is not due to a limitation of the supply of methionine or cysteine to the fungus. Infect Immun. 1992 Feb;60(2):698–700. doi: 10.1128/iai.60.2.698-700.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Brown K., Friedman H. Differential morphologic and metabolic alterations in permissive versus nonpermissive murine macrophages infected with Legionella pneumophila. Infect Immun. 1992 Aug;60(8):3231–3237. doi: 10.1128/iai.60.8.3231-3237.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Newton C. A., Widen R., Friedman H. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect Immun. 1988 Feb;56(2):370–375. doi: 10.1128/iai.56.2.370-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Newton C., Friedman H. Differing macrophage and lymphocyte roles in resistance to Legionella pneumophila infection. J Immunol. 1992 Jan 15;148(2):584–589. [PubMed] [Google Scholar]
- Yoshida R., Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3998–4000. doi: 10.1073/pnas.75.8.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Imanishi J., Oku T., Kishida T., Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci U S A. 1981 Jan;78(1):129–132. doi: 10.1073/pnas.78.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Urade Y., Tokuda M., Hayaishi O. Induction of indoleamine 2,3-dioxygenase in mouse lung during virus infection. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4084–4086. doi: 10.1073/pnas.76.8.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M., Fennie C. W., Czarniecki C. W. The anti-chlamydial and anti-proliferative activities of recombinant murine interferon-gamma are not dependent on tryptophan concentrations. J Immunol. 1985 Dec;135(6):4198–4200. [PubMed] [Google Scholar]



