Introduction to the patient and the most frequent orthostatic complaint
The most frequent orthostatic complaint in young people is lightheadedness or dizziness on rapid standing, sometimes from a seated position, most often from a supine position after hours of recumbency(1, 2). The most common complainant is an adolescent in the middle of a growth spurt. Often the patient is already tall or is growing rapidly. Similar complaints are seen among young people of both sexes, particularly in patients of slender build and reduced blood volume (3). The complaints relate to normal physiology shared by people of all ages and have been identified by students of orthostasis in the past (1, 2, 4–6). Typically, there is transient pallor, perhaps some loss of postural tone, blurred vision or even loss of vision (black out, white out, spots), and less commonly, loss of consciousness. Symptoms instantly disappear with recumbency and are reduced or even abolished by standing up more slowly or in stages (7). Symptoms and signs peak soon after standing, in synchrony with a transient and substantial decrease in blood pressure which has its nadir at approximately 8–15 seconds after standing as shown in Figure 1. Heart rate increases as BP falls. These findings can be demonstrated using beat-to-beat finger arterial photoplethysmography (Finapres, Finometer, TNO Amsterdam) (2, 8, 9) or other means for beat-to-beat assessment. The BP decrements are usually not apparent by auscultatory or oscillometric blood pressure measurement, having a far too rapid and fleeting time course to be detected by these methods (10).
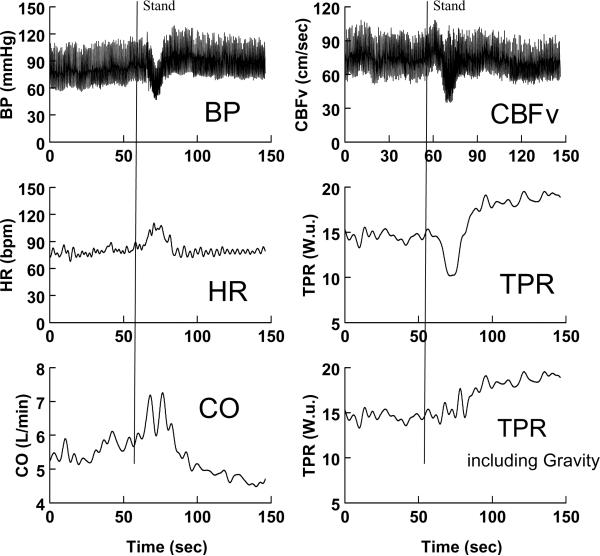
Figure 1.
Rapid standing results in initial orthostatic hypotension in a representative subject. Blood pressure (BP) is shown in the left upper panel, heart rate (HR) in the left middle panel, and cardiac output measured by Modelflow (CO) in the left lower panel. Cerebral blood flow velocity (CBFv) is shown in the right upper panel, total peripheral resistance (TPR) calculated as MAP/CO in the right middle panel, and TPR calculated as (MAP+ ρg<h>)/CO to include the effects of gravity, is shown in the right lower panel. BP, CBFv, and TPR decrease while HR and CO increase transiently during the stand. TPR corrected for gravity increases shortly after BP falls.
Thus, the clinician needs to rely on clinical history and perhaps observation of the patient. The history is that of rapid-onset, short-lived dizziness and other symptoms on rapidly standing upright. If the patient can remain upright or can be held upright, all symptoms abate and disappear within 60 seconds and usually within less than 30 seconds (11). After recovery, the patient is usually entirely well and is free of subsequent fainting and of symptoms of orthostatic symptoms. The patient is otherwise entirely healthy. This stands in contrast with the clinical entity of instantaneous orthostatic hypotension (INOH) described by Tanaka and coworkers (2) in which recovery from hypotension was prolonged or ineffectual. Many sufferers report successfully holding themselves upright until the unpleasant sensations pass. There appears to be no relationship between this transient hypotension and the degree of orthostatic tolerance demonstrated in a laboratory setting in normal young subjects (9). These studies should be cautiously interpreted because they excluded subjects with known OI. However, they do demonstrate that IOH need not cause more pernicious forms of OI. This short-lived hypotension is different from simple faint, in which maintaining the patient in the upright position makes the faint persistent and the patient often becomes much worse, clinically (12).
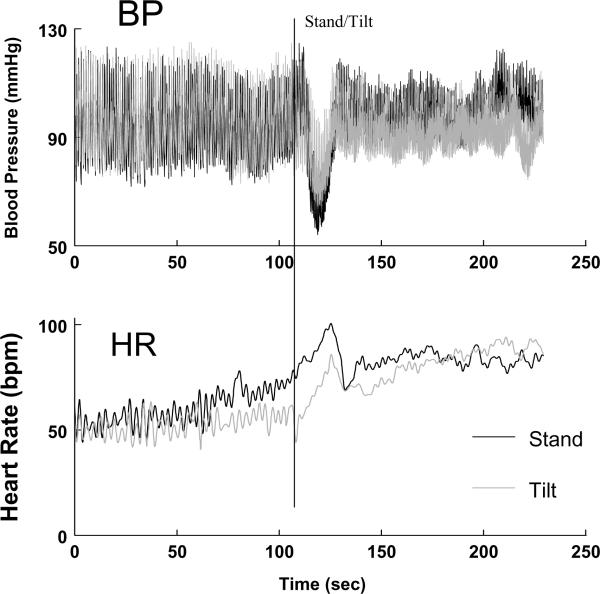
Can it occur on a tilt table? We often observe the phenomenon as tilt commences. However, the decrease in BP is generally of smaller magnitude compared with standing. This is shown in Figure 2 in the same subject who had standing and upright tilt to 70° performed separately on the same morning. The magnitude of BP decrease may depend on the rapidity of upright tilt. Also, during active standing, alterations in BP and HR are more closely related to skeletal muscle activity, abdominal compression and responsive reflex changes (13). In addition, there is an anticipatory increase in both heart rate and blood pressure seconds prior to the stand which is absent during the tilt. This is due to a phenomenon called “central command” in which higher neurological centers engage the autonomic nervous system in order to prepare the body for exercise (14).
Figure 2.
Rapid standing (black lines) is compared with upright tilt (gray lines) to 70° in the same subject. The upper panel shows changes in blood pressure (BP) and the lower panel shows changes in heart rate (HR). There is a greater fall in BP and rise in HR with standing compared with tilt. Also, both BP and HR increase in anticipation of standing - a phenomenon called “central command”.
Initial Orthostatic Hypotension (IOH)
Initial orthostatic hypotension (IOH), aptly named by Wieling and other students of orthostatic intolerance because of early, transient, orthostatic (standing up) hypotension (1, 4, 6, 15, 16) which can be substantial and symptomatic. However, unlike true orthostatic hypotension, with its relentlessly progressive fall in blood pressure while the subject remains upright, IOH is short-lived and blood pressure recovery always occurs. As defined by consensus (17), true orthostatic hypotension (OH) is a sustained reduction of systolic blood pressure of at least 20 mm Hg or diastolic blood pressure of at least 10 mm Hg within 3 minutes of standing or upright tilting to 60° or more. On one hand, true orthostatic hypotension is often associated with autonomic failure or severe autonomic dysfunction which can be life threatening (18). On the other hand, IOH is associated with autonomic integrity and usually has few, if any, consequences. In those of slight build and small blood volume, mean arterial pressure may decrease by nearly 50% (Figure 1) with comparable changes in cerebral blood flow leading to unconsciousness. Such extremes are relatively rare (1/100 young patients seen in our syncope center over last 10 years), but can occur and require medical attention. However, an estimated 50% of adolescents and somewhat fewer young patients in their twenties, experience this difficulty during the first half of life. IOH, again, becomes common and more dangerous in the elderly, especially those patients taking vasoactive medications or those patients with medical problems placing them at increased risk (6). Dehydration is also a potentiating factor. Otherwise, in the young, IOH is evanescent, improves with time as teens age, and can be clinically remediated through preventative or responsive aversive maneuvers (6, 15, 19). IOH is almost always due to normal rather than abnormal physiology. There is no autonomic dysfunction. Merely a [normal] delay in compensation for gravitational loading during postural change (20).
But is it Orthostatic Intolerance?
It is, by definition, but it is not autonomic dysfunction. Orthostatic intolerance (OI) is defined as the development of symptoms and signs when upright that are relieved by recumbence (21, 22). Even so, other forms of brief OI are facts of everyday life and may be thought to be normal aspects of life which are not necessarily harmful. For example, the experiences of postural lightheadedness during states of dehydration or fever are universal. More life-affecting forms of orthostatic intolerance are also common and include simple postural faint (vasovagal faint, neurocardiogenic syncope, reflex syncope, (23, 24)) as a prevalent form of acute orthostatic intolerance in which transient loss of consciousness and postural tone occurs as the result of cerebral hypoperfusion (25). This almost always results from hypotension occurring while upright. Chronic orthostatic intolerance occurs as well and includes the postural tachycardia syndrome (POTS) (26–28), and neurally mediated hypotension (29–31) which may occur alone or as part of chronic fatigue syndrome (32). Postural hyperventilation has also been reported (33). Simple faint and chronic OI are related by the common feature of reduced cardiac filling, and therefore early reflex tachycardia is common to both (34, 35). Estimates of lifetime incidence of at least one fainting episode may reach 40–50% approximately half occurring during childhood (36). Fainting, NMH, POTS, or postural hyperpnea may be provoked in virtually any healthy subject in the laboratory with sufficient central hypovolemia using a tilt table and lower body negative pressure together to profoundly unload the heart (37). Therefore, these are not diseases in a strict sense; autonomic dysfunction is not necessary for their occurrence. Rather, these problems represent circulatory inadequacy in efficiently coping with physiological stressors, with varying response thresholds, in different individuals. Nevertheless, syncope and chronic OI can interfere with quality of life and thus require remediation.
Is Initial Orthostatic Hypotension different from simple faint?
The hallmark of IOH is its immediacy and transient nature, with restoration of blood pressure in less than a minute, although the extent of associated hypotension may be occasionally sufficient to produce transient loss of consciousness. This is distinguished from simple postural faint which is not immediate but occurs after some minutes of upright stance. Also, the young patient with IOH will usually recover normal blood pressure even if upright stance is artificially maintained by propping the patient up. This is not at all the case for simple faint which, if standing is artificially maintained, progresses to complete loss of blood pressure and often severe bradycardia or asystole (38) with potentially pernicious outcome (12). Finally, and characteristically, simple postural faint in the young is almost always vasovagal faint which means that after a sufficient time upright, there is a hypotension (vasodilation) followed almost immediately by vagally mediated relative bradycardia (39).
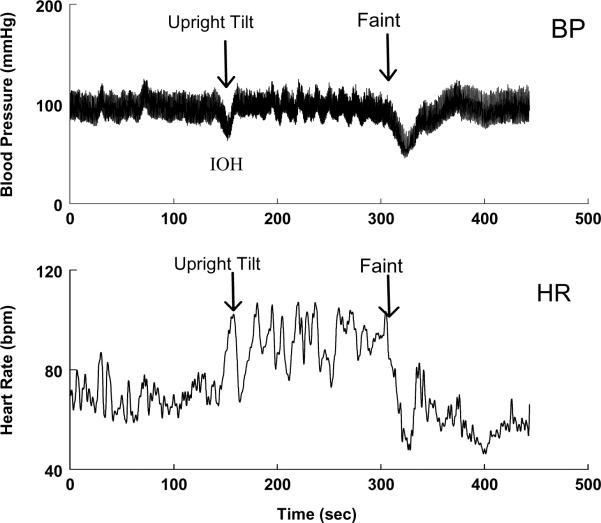
This contrasts with observations during IOH. Figure 3 shows blood pressure and heart rate in a patient with a history of postural fainting who upon upright tilt has IOH, recovers, and then goes on to suffer a typical vasovagal episode. It is clear that the hypotension of IOH is accompanied by tachycardia, and the delayed hypotension of the vasovagal faint is accompanied by bradycardia. In the first instance (IOH), tachycardia is a reflex response to hypotension. In the second instance (vasovagal faint), early upright reflex tachycardia does occur in relation to blood volume shifts in the upright position (40). However, subsequent hypotension is associated with bradycardia which would seem physiologically inappropriate. The inappropriate bradycardia reflects baroreflex failure at faint; the tachycardia of IOH reflects physiologically appropriate baroreflex function.
Figure 3.
Initial orthostatic hypotension at the onset of upright tilt is compared with vasovagal faint in a representative patient. The upper panel shows blood pressure (BP) and the lower panel shows heart rate (HR). HR increases with IOH but decreases with fainting.
A few words about Physiology
Supine Physiology
While supine, arterial blood and venous reservoirs are at the same height. There is no net gravitational force acting on blood. Neurovascular mechanisms, such as peripheral vasoconstriction and venoconstriction, local and humoral vasoactive factors (e.g. epinephrine, angiotensin-II, vasopressin, nitric oxide) are in their tonic resting conditions. The supine arterial vasculature is relatively vasodilated compared with its state when upright. While supine, the dependent venous pools are relatively underfilled having cranially redistributed blood while supine (41, 42).
Compensated Upright Physiology
Upright posture requires rapid circulatory and neurologic compensation in order to maintain blood pressure and consciousness. If these are ineffective or if there is insufficient blood volume, the location of the brain above the heart and the presence of large venous reservoirs below the heart causes cardiac filling and blood pressure to decrease rapidly with consequent cerebral malperfusion and loss of consciousness (42). This describes the steady condition that is impaired in simple faint and autonomic failure.
The transition from supine to upright
The transition to the steady upright condition, that is, from supine to standing up, is not included in this description. The physiology changes radically when someone stands up. An ensemble of anti-gravity, anti-orthostatic physiological responses are evoked during standing up including the skeletal muscle pump (43) and arterial and cardiopulmonary baroreflexes (44, 45). Actual vasodilation does not occur (46), although the literature suggests that the more vasodilated you are prior to orthostasis, the greater the initial hypotensive effect (20, 47). Observations of increasing vasodilation are based on calculated total peripheral resistance (TPR) = MAP/CO. As MAP decreases transiently, CO increases as shown in Figure 1. This occurs before effective vasoconstriction can occur.
The traditional calculation of TPR fails to factor in the abrupt onset of an additional force, gravity, which effectively propels blood caudally (48). The initial force of gravity is not effectively counterbalanced by skeletal muscle pumping because the leg veins are initially underfilled for the upright position. Later, during steady state standing, the effect of gravity is compensated by a pressure difference equal and opposite to gravitational force (ρgh) conferring mechanical equilibrium. Such equilibrium is initially absent upon rapid standing (49). Estimates of the initial pressure gradient for the subject of Figure 1 taking into consideration the average height of the hemostatic column from heart to splanchnic bed (approximately 20 cms) and from heart to mid thigh (approximately 80 cms) yields an average estimated height <h> of 50 cms of blood. This corresponds to a pressure of ρg<h> or roughly 0.776 mmHg /cm blood *50cms = 39 mmHg for the subject of Figure 1. When TPR is recalculated as TPR = (MAP+ ρg<h>)/CO the apparent initial decrease in TPR disappears as shown in the bottom right hand panel of Figure 1. This has been aptly described as a “virtual conductance” by Sheriff and others (46, 47). Thus, at least initially, blood is propelled by uncompensated gravity, and is also pumped by the heart in declining amounts as cardiac venous return decreases until vasoconstriction occurs and a mechanical equilibrium is reached. The lungs and pulmonary venous return help to support left ventricular filling but this is unable to maintain BP.
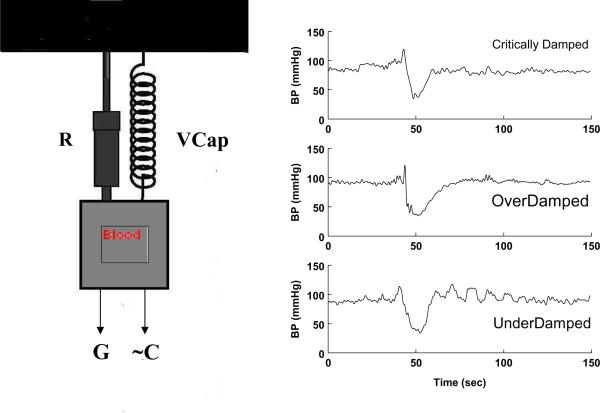
For those technically minded, the effect resembles a periodically driven (pressure generated by cardiac contraction, ~CP), resistance damped (blood vessel vasoconstriction, R) harmonic oscillator upon which a constant force (G) is suddenly applied (50). The characteristic modes of operation of such a system can be found among the responses to standing and appear in Figure 4.
Figure 4.
The right panels show blood pressure responses to rapid standing of 3 separate patients. The top panel, labeled “critically damped” shows a rapid recovery phase, the middle panel shows a slower recovery that can be best fit by two exponential functions and the bottom panel labeled “underdamped” shows an oscillatory BP recovery. These are characteristic modes of a damped harmonic oscillator modeled in the left hand side of the figure. The oscillator originally driven by a periodic cardiac rhythm (~C) upon which is abruptly superimposed the effects of a gravitational force (G). R corresponds to vascular resistance (the damping) and VCap is the vascular capacitance.
Treatment of the young with IOH
Often reassurance is all that is needed, particularly if there is no loss of postural tone and the episodes are brief. Standing up more rapidly comes with youth and departs with age which may account for the maturational diminution of IOH. Rising more slowly may be sufficient therapy. Situations or illnesses that promote a contraction of blood volume can often be treated or avoided. Measures to increase blood volume such as enhanced salt and water intake may be of help. For more debilitating forms of IOH preventiveand treatment options currently favor physical maneuvers. Bilateral handgrip for 15 seconds before rising forestalls IOH by evoking the exercise pressor reflex which can substantially increase blood pressure (11). Lower body muscle contraction while seated (e.g. pumping calf muscles) can also help as a preemptive maneuver. Also, lower body muscle tensing of legs, buttocks and abdomen particularly attenuates the transient arterial blood pressure decrease once standing has occurred (15) and can be potentiated by handgrip. Recumbence and squatting are general measures used to remediate all forms of orthostatic intolerance. I have never had a patient who required more than physical aversive measures for IOH.
Summary
Initial orthostatic hypotension is a normal short-lived response to rapid standing caused by a sudden increase of gravitational force. It is not a form of autonomic disease or maladaptation and is distinct in signs and symptoms from vasovagal faint. In some young subjects, the extent of hypotension may be large enough to produce symptoms. If symptoms impair the quality of life treatment in the form of aversive maneuvers should be employed.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (grants RO1HL074873, RO1HL087803, and R21HL091948).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 2007;112:157–65. doi: 10.1042/CS20060091. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka H, Yamaguchi H, Matushima R, Tamai H. Instantaneous orthostatic hypotension in children and adolescents: a new entity of orthostatic intolerance. Pediatr Res. 1999;46:691–6. doi: 10.1203/00006450-199912000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Owens PE, Lyons SP, O'Brien ET. Arterial hypotension: prevalence of low blood pressure in the general population using ambulatory blood pressure monitoring. J Hum Hypertens. 2000;14:243–7. doi: 10.1038/sj.jhh.1000973. [DOI] [PubMed] [Google Scholar]
- 4.Borst C, van Brederode JF, Wieling W, van Montfrans GA, Dunning AJ. Mechanisms of initial blood pressure response to postural change. Clin Sci (Lond) 1984;67:321–7. doi: 10.1042/cs0670321. [DOI] [PubMed] [Google Scholar]
- 5.Lewis T. A lecture on vasovagal syncope and the carotid sinus mechanism with comments on Gowers's and Nothangel's syndrome. BMJ. 1932;1:873–876. doi: 10.1136/bmj.1.3723.873. Ref Type: Journal (Full) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wieling W, Harms MP, Kortz RA, Linzer M. Initial orthostatic hypotension as a cause of recurrent syncope: a case report. Clin Auton Res. 2001;11:269–70. doi: 10.1007/BF02298960. [DOI] [PubMed] [Google Scholar]
- 7.Postural hypotension. Faintness upon standing. Mayo Clin Health Lett. 1999;17:6. [PubMed] [Google Scholar]
- 8.Tanaka H, Thulesius O, Borres M, Yamaguchi H, Mino M. Blood pressure responses in Japanese and Swedish children in the supine and standing position. Eur Heart J. 1994;15:1011–9. doi: 10.1093/oxfordjournals.eurheartj.a060622. [DOI] [PubMed] [Google Scholar]
- 9.Thomas KN, Cotter JD, Galvin SD, Williams MJ, Willie CK, Ainslie PN. Initial orthostatic hypotension is unrelated to orthostatic tolerance in healthy young subjects. J Appl Physiol. 2009;107:506–17. doi: 10.1152/japplphysiol.91650.2008. [DOI] [PubMed] [Google Scholar]
- 10.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 11.Clarke DA, Medow MS, Taneja I, Ocon AJ, Stewart JM. Initial orthostatic hypotension in the young is attenuated by static handgrip. J Pediatr. 2010;156:1019–22. 1022. doi: 10.1016/j.jpeds.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folino AF, Buja GF, Martini B, Miorelli M, Nava A. Prolonged cardiac arrest and complete AV block during upright tilt test in young patients with syncope of unknown origin--prognostic and therapeutic implications. Eur Heart J. 1992;13:1416–21. doi: 10.1093/oxfordjournals.eurheartj.a060076. [DOI] [PubMed] [Google Scholar]
- 13.Pancheva MV, Panchev VS, Suvandjieva AV. Improved orthostatic tolerance by leg crossing and muscle tensing: indisputable evidence for the arteriovenous pump existence. J Appl Physiol. 2006;101:1271–2. doi: 10.1152/japplphysiol.00434.2006. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell JH. Cardiovascular control during exercise: central and reflex neural mechanisms. Am J Cardiol. 1985;55:34D–41D. doi: 10.1016/0002-9149(85)91053-7. [DOI] [PubMed] [Google Scholar]
- 15.Krediet CT, Go-Schon IK, Kim YS, Linzer M, Van Lieshout JJ, Wieling W. Management of initial orthostatic hypotension: lower body muscle tensing attenuates the transient arterial blood pressure decrease upon standing from squatting. Clin Sci (Lond) 2007;113:401–7. doi: 10.1042/CS20070064. [DOI] [PubMed] [Google Scholar]
- 16.Wieling W, Shepherd JT. Initial and delayed circulatory responses to orthostatic stress in normal humans and in subjects with orthostatic intolerance. Int Angiol. 1992;11:69–82. [PubMed] [Google Scholar]
- 17.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology 1996. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 18.Smit AA, Halliwill JR, Low PA, Wieling W. Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol. 1999;519(Pt 1):1–10. doi: 10.1111/j.1469-7793.1999.0001o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krediet CT. Initial orthostatic hypotension in a 37-year old horse rider. Clin Auton Res. 2002;12:404. doi: 10.1007/s10286-002-0062-6. [DOI] [PubMed] [Google Scholar]
- 20.Stewart JM. Transient orthostatic hypotension is common in adolescents. J Pediatr. 2002;140:418–24. doi: 10.1067/mpd.2002.122643. [DOI] [PubMed] [Google Scholar]
- 21.Robertson D, Biaggioni I, Ertl AC, Robertson RM, Diedrich A, Blakely RD, et al. Orthostatic intolerance: emerging genetic and environmental etiologies. J Gravit Physiol. 1999;6:51–4. [PubMed] [Google Scholar]
- 22.Stewart JM. Orthostatic intolerance in pediatrics. J Pediatr. 2002;140:404–11. doi: 10.1067/mpd.2002.122727. [DOI] [PubMed] [Google Scholar]
- 23.Grubb BP. Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation. 2005;111:2997–3006. doi: 10.1161/CIRCULATIONAHA.104.482018. [DOI] [PubMed] [Google Scholar]
- 24.Sutton R, Benditt D, Brignole M, Moya A. Syncope: diagnosis and management according to the 2009 guidelines of the European Society of Cardiology. Pol Arch Med Wewn. 2010;120:42–7. [PubMed] [Google Scholar]
- 25.Thomas KN, Galvin SD, Williams MJ, Willie CK, Ainslie PN. Identical pattern of cerebral hypoperfusion during different types of syncope. J Hum Hypertens. 2009 doi: 10.1038/jhh.2009.93. [DOI] [PubMed] [Google Scholar]
- 26.Raj SR. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–7. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Stewart JM. Chronic orthostatic intolerance and the postural tachycardia syndrome (POTS) J Pediatr. 2004;145:725–30. doi: 10.1016/j.jpeds.2004.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bou-Holaigah I, Rowe PC, Kan J, Calkins H. The relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA. 1995;274:961–7. [PubMed] [Google Scholar]
- 30.Goldstein DS, Eldadah B, Holmes C, Pechnik S, Moak J, Sharabi Y. Neurocirculatory abnormalities in chronic orthostatic intolerance. Circulation. 2005;111:839–45. doi: 10.1161/01.CIR.0000155613.20376.CA. [DOI] [PubMed] [Google Scholar]
- 31.Rowe PC, Bou-Holaigah I, Kan JS, Calkins H. Is neurally mediated hypotension an unrecognised cause of chronic fatigue? Lancet. 1995;345:623–4. doi: 10.1016/s0140-6736(95)90525-1. [DOI] [PubMed] [Google Scholar]
- 32.Rowe PC. Orthostatic intolerance and chronic fatigue syndrome: New light on an old problem. J Pediatr. 2002;140:387–9. doi: 10.1067/mpd.2002.124318. [DOI] [PubMed] [Google Scholar]
- 33.Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol. 2006;291:H904–H913. doi: 10.1152/ajpheart.01359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart JM. Postural tachycardia syndrome and reflex syncope: similarities and differences. J Pediatr. 2009;154:481–5. doi: 10.1016/j.jpeds.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taneja I, Medow MS, Glover JL, Raghunath NK, Stewart JM. Increased vasoconstriction predisposes to hyperpnea and postural faint. Am J Physiol Heart Circ Physiol. 2008;295:H372–H381. doi: 10.1152/ajpheart.00101.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, et al. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC) Eur Heart J. 2009;30:2631–71. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeLorier P, Klein GJ, Krahn A, Yee R, Skanes A, Shoemaker JK. Combined head-up tilt and lower body negative pressure as an experimental model of orthostatic syncope. J Cardiovasc Electrophysiol. 2003;14:920–4. doi: 10.1046/j.1540-8167.2003.03065.x. [DOI] [PubMed] [Google Scholar]
- 38.Grubb BP, Gerard G, Roush K, Temesy-Armos P, Elliott L, Hahn H, et al. Differentiation of convulsive syncope and epilepsy with head-up tilt testing. Ann Intern Med. 1991;115:871–6. doi: 10.7326/0003-4819-115-11-871. [DOI] [PubMed] [Google Scholar]
- 39.Morillo CA, Eckberg DL, Ellenbogen KA, Beightol LA, Hoag JB, Tahvanainen KU, et al. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation. 1997;96:2509–13. doi: 10.1161/01.cir.96.8.2509. [DOI] [PubMed] [Google Scholar]
- 40.Rowell LB. Human Cardiovascular Control. Oxford University Press; NY, NY.: 1993. [Google Scholar]
- 41.Maw GJ, Mackenzie IL, Taylor NA. Redistribution of body fluids during postural manipulations. Acta Physiol Scand. 1995;155:157–63. doi: 10.1111/j.1748-1716.1995.tb09960.x. [DOI] [PubMed] [Google Scholar]
- 42.Rowell LB. Human Cardiovascular Control. Oxford University Press; NY, NY.: 1993. The Passive Effects of Gravity. [Google Scholar]
- 43.Ten Harkel AD, Van Lieshout JJ, Wieling W. Effects of leg muscle pumping and tensing on orthostatic arterial pressure: a study in normal subjects and patients with autonomic failure. Clin Sci (Colch) 1994;87:553–8. doi: 10.1042/cs0870553. [DOI] [PubMed] [Google Scholar]
- 44.Jacobsen TN, Morgan BJ, Scherrer U, Vissing SF, Lange RA, Johnson N, et al. Relative contributions of cardiopulmonary and sinoaortic baroreflexes in causing sympathetic activation in the human skeletal muscle circulation during orthostatic stress. Circ Res. 1993;73:367–78. doi: 10.1161/01.res.73.2.367. [DOI] [PubMed] [Google Scholar]
- 45.Victor RG, Mark AL. Interaction of cardiopulmonary and carotid baroreflex control of vascular resistance in humans. J Clin Invest. 1985;76:1592–8. doi: 10.1172/JCI112142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halliwill JR. Virtual conductance, real hypotension: what happens when we stand up too fast? J Appl Physiol. 2007;103:421–2. doi: 10.1152/japplphysiol.00544.2007. [DOI] [PubMed] [Google Scholar]
- 47.Sheriff DD, Nadland IH, Toska K. Hemodynamic consequences of rapid changes in posture in humans. J Appl Physiol. 2007;103:452–8. doi: 10.1152/japplphysiol.01190.2006. [DOI] [PubMed] [Google Scholar]
- 48.Hill L. The influences of the force of gravity on the circulation of the blood. J Physiol (London) 1951;18:15–53. doi: 10.1113/jphysiol.1895.sp000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark JR, Hooker DR, Weed LH. The hydrostatic factor in venous pressure measurements. American Journal of Physiology. 1932;109:166–177. Ref Type: Journal (Full) [Google Scholar]
- 50.Klepner D, Kolekow RJ. An Introduction to Mechanics. Cambridge University press; Cambridge, UK: 2010. The Harmonic Oscillator. The Forced harmonic oscillator; pp. 414–38. [Google Scholar]