Abstract
Adult mammalian brains are capable of some structural plasticity. Although the basic cellular mechanisms underlying learning and memory are being revealed, extrinsic factors contributing to this plasticity remain unspecified. White-footed mice (Peromyscus leucopus) are particularly well suited to investigate brain plasticity because they show marked seasonal changes in structure and function of the hippocampus induced by a distinct environmental signal, viz., photoperiod (i.e., the number of hours of light/day). Compared to animals maintained in 16 h of light/day, exposure to 8 h of light/day for 10 weeks induces several phenotypic changes in P. leucopus, including reduction in brain mass and hippocampal volume. To investigate the functional consequences of reduced hippocampal size, we examined the effects of photoperiod on spatial learning and memory in the Barnes maze, and on long term potentiation (LTP) in the hippocampus, a leading candidate for a synaptic mechanism underlying spatial learning and memory in rodents. Exposure to short days for 10 weeks decreased LTP in the Schaffer collateral-CA1 pathway of the hippocampus and impaired spatial learning and memory ability in the Barnes maze. Taken together, these results demonstrate a functional change in the hippocampus in male white-footed mice induced by day length.
1. INTRODUCTION
One important goal of neuroscience is to understand the mechanisms underlying brain plasticity. Toward that end, substantial research has been conducted using in vivo and ex vivo chemical and genetic manipulations of standard laboratory rodent research models (Fisher, 1997; Wells and Carter, 2001; Smale et al., 2005). However, natural selection has produced many vertebrate species that display plasticity in physiology and behavior across seasons as adaptive mechanisms for survival. Seasonal changes in physiology and behavior, driven by the annual cycle of changing day length (photoperiod), presumably evolved to allocate resources among competing energetically expensive physiological processes in individuals living outside of tropical latitudes (Nelson et al., 2010). Among these energy-coping tradeoffs are morphological and functional changes in the brain which have been studied extensively in non-mammalian vertebrates such as reptiles (Delgado-Gonzalez et al., 2008), fish (Zhang et al., 2009; Walton et al., 2010), and birds (Nottebohm, 2004; Ball and Balthazart, 2010). Most photoperiodic brain plasticity studies have been conducted in birds, focusing primarily on brain regions comprising the song control system (Meitzen and Thompson, 2008; Ball and Balthazart, 2010), and the hippocampus (Sherry and Hoshooley, 2010). Compared to birds, few mammalian models of photoperiodic brain plasticity exist (Hofman and Swaab, 2002). Studies of naturally-occurring examples of brain plasticity may provide novel insights into the regulatory mechanisms underlying such processes.
White-footed mice (Peromyscus leucopus), are photoperiodic rodents indigenous to the eastern and southern United States and eastern Mexico (King, 1968). This species has evolved a suite of adaptive responses to survive the harsh conditions of winter, including reducing the size of the reproductive system, as well as the brain, that are induced by exposure to short days (Pyter et al., 2005a). Changes in brain morphology in P. leucopus following exposure to short day lengths are accompanied by impaired spatial learning and memory in the Morris water maze (Pyter et al., 2005a; Pyter et al., 2006; Workman et al., 2009).
Spatial learning and memory is correlated with hippocampal long-term potentiation (LTP; Shapiro and Eichenbaum, 1999; Lynch, 2004), such that LTP is induced in the hippocampus during learning (Whitlock et al., 2006), inhibition of LTP impairs performance in spatial learning tasks (Morris et al., 1986), and LTP maintenance in the hippocampus is required for retention of spatial memory (Pastalkova et al., 2006). LTP, a measure of increased efficiency of synaptic transmission following high-frequency stimulation (Bliss and Gardner-Medwin, 1973), is the strongest candidate for a synaptic mechanism by which new memories are formed and stored in the brain (Bliss and Collingridge, 1993; Malenka, 2003; Lynch, 2004). Although a complete understanding of how LTP translates into memory storage remains unspecified, to better understand the mechanisms underlying the short-day impairment of spatial learning and memory, we examined the role of photoperiod on in vitro hippocampal LTP in P. leucopus. Additionally, to confirm and extend previous findings on spatial learning and memory in P. leucopus using the Morris water maze, we examined the role of photoperiod on spatial learning and memory in the Barnes maze, a dry-land, and more ecologically-relevant, alternative to the Morris water maze.
2. Experimental Procedures
2.1 Animals
Thirty four adult male Peromyscus leucopus, from our breeding colony at the Ohio State University, were singly housed upon weaning and held in long days (LD, 16L:8D) until sexual maturity (>60 days). Upon reaching adulthood, mice were randomly assigned to either LD (n = 15) or short days (SD, 8L:16D; n = 19). Mice were maintained in photoperiod for 10 weeks to establish photoperiod-induced changes prior to testing (Pyter et al., 2005a; Workman et al., 2009). Eighteen mice were used for LTP analysis (LD n = 9; SD n = 9) and the remainder were used for behavioral testing in the Barnes maze and assessment of reproductive tissue mass (LD n = 6; SD n = 10). Two mice that failed to regress their gonads in response to SD exposure were excluded from Barnes maze and reproductive tissue mass analysis.
Mice were housed in standard polycarbonate cages (32×18×14 cm), maintained at constant temperature (21 ± 4°C) and relative humidity (50 ± 5%), provided ad libitum access to filtered tap water and food (Harlan Teklad 8640, Indianapolis, IN, USA), and received care from the Ohio State University Laboratory Animal Resource staff for the duration of the study. All procedures were approved by the Ohio State University Institutional Animal Care and Use Committee and comply with guidelines established by the National Institutes of Health published in Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
2.2 Long-term potentiation
Late during the light phase, to coincide with the time of behavioral testing (see below), transverse hippocampal slices (400 μm) were prepared from male Peromyscus that had been exposed to LD (n = 9) and SD (n = 9). Slices were maintained at 28°C in an incubation chamber bubbled with mixture of 95% O2 and 5% CO2. Following 90 min incubation, one slice was then transferred to a recording chamber affixed to a microscope (model E600FN, Nikon) with a custom stage (Syskiyou Instruments, Grants Pass, OR) with continuous perfusion (2 ml/min) of oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM) 124 NaCl, 4 MgSO4, 4 KCl, 1.0 Na2HPO4, 4 CaCl2, 26 NaHCO3, 10 D-glucose. A bipolar stimulating electrode (67 μm, NiCr) was placed in the stratum radiatum to stimulate the Schaffer collateral-CA1 pathway. Field excitatory post-synaptic potentials (fEPSPs) were recorded (model 2400, AM Systems) with a glass electrode (1-2 MΩ) filled with aCSF. Input/output curves were established by recording fEPSPs in response to different stimulating intensity (from 20 to 100 μA). Baseline synaptic responses were recorded with a test stimulus delivered at 0.033 Hz (0.1 ms pulse duration). The stimulus strength was adjusted so that it gave rise to fEPSPs with slope values between 30-40% of the maximal response recorded during the input/output measurements (Selcher et al., 2003; Nguyen, 2006). Following a 30-min recording of stable responses (less than 10% variation), two trains of tetanus stimuli (100 Hz for 1 sec, 5 min apart) were delivered to induce long-term potentiation (LTP). The mean percent change of fEPSPs slope was calculated from one slice from each animal. The slope calculations for each animal were normalized to the mean of the control responses recorded during the 30 min prior to tetanus stimulation.
2.3 Barnes maze
The Barnes maze, (122 cm diameter) with 18 escape holes (9.5 cm) placed every 20° around the perimeter (ENV-563-R, MedAssociates, St. Albans, VT, USA), was completely surrounded with a 60 cm high white polycarbonate barrier to prevent escape. The blind escape holes were blocked by black panels, and the target escape hole was visually the same as the blind holes, but contained a black escape box (38.7 × 12.1 × 14.2 cm). Distinct visual cues (black 2 dimensional geometric shapes, 20 – 25 cm) were attached to the upper edge of the surround at the 4 compass points and visual cues distal to the maze were present along the walls of the room. Light intensity measured at the maze surface was 1200 lux, which was approximately twice the average intensity of illumination measured 100 cm from the floor in the vivarium housing rooms (LD=599 lux, SD=600 lux).
To avoid disruption of photic circadian cues, all testing was performed late in the light phase and animals were returned to their vivarium rooms prior to the onset of darkness. Testing consisted of 5 days of acquisition training followed by a single probe trial 24 h after the last training trial. Each acquisition day consisted of one session/animal, 3 trials per session, with an inter-trial interval of 5 min. For acquisition training, all mice were brought into the testing room and allowed to acclimate for 30 min before the start of testing. Mice were moved from their home cage to the testing arena in an opaque plastic beaker covered with a small net to avoid direct handling. Each trial consisted of carefully placing the mouse in the center of the maze from the opaque plastic beaker. The mouse was allowed to search for the escape box for 120 s. If the mouse had not found the escape box by 120s, it was gently guided to it using a small net. The mouse was allowed to remain in the escape box for 60 s, and then returned to its home cage for a minimum of 45 s. To minimize olfactory cues, the surface of the maze was cleaned with 70% EtOH at the conclusion of testing of each mouse, and each day the maze was rotated 90° counter clockwise, with the escape box location and location of visual cues remaining constant throughout testing. The probe trial consisted of a single 90 s trial with the former escape box removed and replaced with a blind box. Twenty-four hours after the conclusion of behavioral testing, animals were killed and reproductive tissues were collected and weighed.
All behavior was recorded and scored using The Observer software (XT 8.0; Noldus, Leesburg, VA, USA) by an observer uninformed as to the treatment of the mice. For training trials, latency to escape and number of errors were recorded. An error was defined as an investigation of a blind escape hole where the entire head of the mouse broke the plane of the edge of the escape hole. For the probe trial, latency to escape hole, number of errors, and time in quadrant of escape hole were measured. Learning criterion was defined as making an average of ≤5 errors for 3 trials in one day (Bredy et al., 2004).
2.4 Statistical Analyses
Repeated measures ANOVAs were used to compare Barnes maze performance across days and LTP across time between LD and SD groups. Within days, a priori comparisons of photoperiod were conducted using two-tailed Student's t-tests. Two-tailed Student's t-tests were also used for other comparisons of behavioral and reproductive tissue mass data between LD and SD groups. All comparisons were considered statistically significant when p < 0.05.
3. RESULTS
3.1 Short days reduce body and reproductive tissue mass
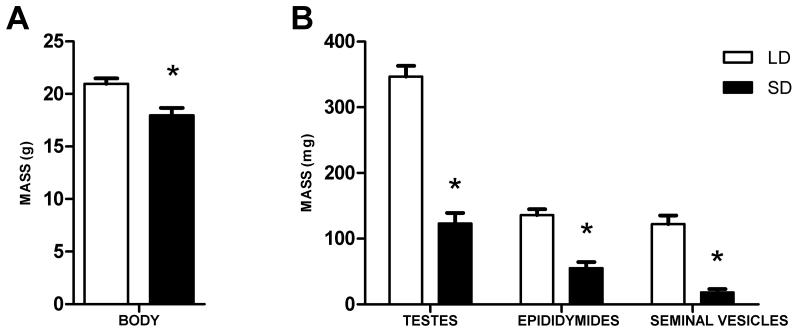
Exposure to SD reduced body mass (t(12) = 3.142, p < 0.05; Figure 1A), mass of paired testes (t(12) = 9.620, p < 0.001), epididymides (t(12) = 6.080, p < 0.001), and seminal vesicles (t(12) = 8.460, p < 0.001; Figure 1B).
Figure 1.
Photoperiodic (A) body and (B) reproductive tissue responses in P. leucopus to 11 week SD exposure. Long day (LD) n = 6, short day (SD) n = 8. *p < 0.05.
3.2 Short days impair Barnes maze performance
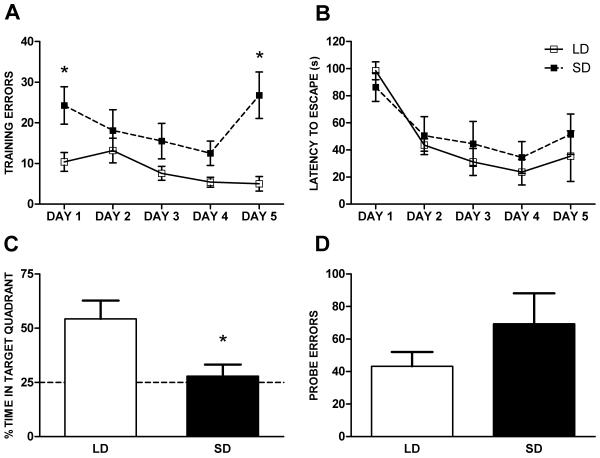
Compared to LD mice, exposure to SD increased errors in finding the escape hole across days during acquisition training (F(1,7) = 6.775, p < 0.05; Figure 2A). There was no effect of photoperiod on latency to escape the Barnes maze across days (F(1,7) = 0.230, p > 0.05; Figure 2B). However, both groups of mice displayed learning as the latency to escape the Barnes maze decreased over days of training (SD: F(1,4)= 5.231, p < 0.05; LD F(1,4)= 10.996, p < 0.05; Figure 2B). SD exposure decreased the time spent in the quadrant of the former escape hole during the probe trial (t(12) = 2.774, p <0.05), so that the SD mice spent no more time in the target quadrant than expected by chance (25%, Figure 2C), without significantly increasing total errors during the probe trial (t(12) = −1.727, p = 0.11; Figure 2D).
Figure 2.
Photoperiod induced deficits in spatial learning and memory in the Barnes maze after 10 week SD exposure. A, Escape errors by day during training. B, Latency to escape maze by day of training. C, Percent time in target quadrant during 90 s probe trial. The dashed line indicates the expected time in the target quadrant by chance (25%). D, Errors (incorrect escape hole choice) during 90 s probe trial. Long day (LD) n = 6, short day (SD) n = 8. *p < 0.05.
3.3 Short days impair LTP
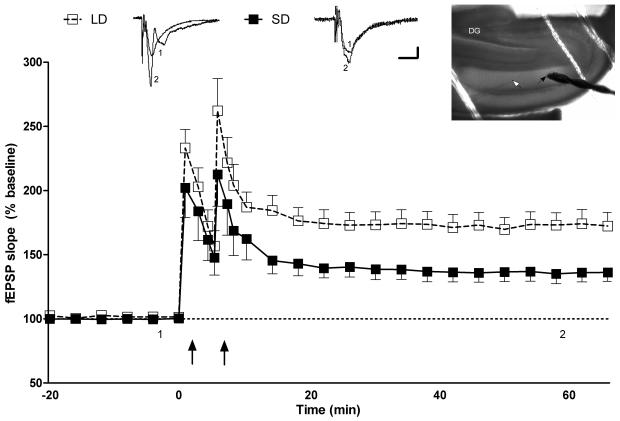
Two trains of tetanic stimuli induced long-term potentiation of fEPSP slope in the Schaffer Colleteral-CA1 pathway of hippocampal slices 60 minutes post-tetanus in LD (n = 9, 172.4 ± 10.6%). LTP was also induced at a significantly decreased level in SD hippocampal slices 60 min post-tetanus (n = 9, 136.2 ± 7.0%; Figure 3). Compared to LD exposed mice, SD exposure decreased the level of LTP induced at all time points after the paired tetanus stimuli until the end of the experiment (F(1,15)= 6.682, p < 0.05; Figure 3).
Figure 3.
Photoperiodic impairment of long-term potentiation of fEPSPs in the Schaffer Colleteral-CA1 pathway of hippocampal slices after 10 wk exposure to SD. Upper left and middle; representative traces of fEPSPs (1) pre-tetanus and (2) 50 min post-tetanus. Calibration bar: 0.2mV; 10 ms. Upper right; location of stimulating (black arrow) and recording (white arrow) electrodes. Lower panel; comparison of LTP between LD (open box) and SD (black box) mice. Tetanus stimulation was delivered at the time indicated by two arrows. Long day (LD) n = 9, short day (SD) n = 9.
4. DISCUSSION
The present study establishes that short days decrease the amplitude of LTP in the CA1 region of the hippocampus in vitro and impair spatial learning and memory in the Barnes maze. The observed behavioral impairment in short days confirms and extends the previous reports that short days impair spatial learning and memory in the Morris water maze in male P. leucopus (Pyter et al., 2005a). Additionally, the decrease in LTP in SD exposed male P. leucopus provides a functional correlate of the short day induced reduction in hippocampal volume and dendritic spine density (Pyter et al., 2005a). It is well established that LTP is associated with spatial learning and memory, and thus our data reveal that LTP can be modified by day length.
Day length is encoded physiologically by the nightly duration of pineal melatonin secretion so that short days are encoded by a relatively long duration of elevated melatonin secretion, whereas long days are encoded by a relatively short duration of sustained melatonin secretion (Carter and Goldman, 1983; Bittman and Karsch, 1984; reviewed in: Bartness et al., 1993; Reiter, 1993; Prendergast et al., 2009). Although the precise role of melatonin on hippocampal function remains obscure, several studies have indicated that pharmacological treatment with melatonin blocks LTP in the CA1 region of the hippocampus (Feng et al., 2002; Wang et al., 2005; Ozcan et al., 2006; Talaei et al., 2010), as well as impairs spatial learning and memory performance (Collins and Davies, 1997; Feng et al., 2002; Cao et al., 2009). Thus, our data appear to be consistent with the attenuating effects of melatonin on LTP and spatial learning and memory, i.e., SD corresponds to extended melatonin exposure. However, one critical difference between the present study and previous studies is the assessment of naturalistic melatonin rhythms (current study) compared to the direct effects of exogenous melatonin on LTP and learning and memory. Although melatonin was not assayed in the current study, it is the duration of pineal melatonin excretion, but not absolute melatonin concentration at a specific time point, that is the critical signal transducer underlying the short-day photoperiodic responses in P. leucopus (Dowell and Lynch, 1987; Carlson et al., 1989). Inbred laboratory rodents have been selected to be nonresponsive to photoperiod and many strains have blunted or absent pineal melatonin rhythms (Ebihara et al., 1986; Goto et al., 1989; Stehle et al., 2002; Simonneaux et al., 2006). Thus, results from inbred laboratory strains of mice and rats should be interpreted with caution and studies using naturally selected species can produce reliable and valid data on this issue.
Melatonin may also have indirect effects on LTP as demonstrated in the current study. Hippocampal slices were prepared and tested during the light phase when pineal melatonin production is at its nadir, and thus the photoperiodic differences reported here persist in the absence of circulating or exogenously administered melatonin. Furthermore, it has been demonstrated that circadian rhythms in CA1 LTP and neural excitability exist independent of melatonin (Chaudhury et al., 2005). Thus, it is possible that the effects of photoperiod we report here may not be melatonin-dependent, but could be a result of altered circadian rhythms induced by changes in photoperiod. Further experiments using pinealectomized male P. leucopus are necessary to test this hypothesis.
Underlying the photoperiodic reduction of brain mass and function in this species are reductions in the mass of reproductive tissues and testosterone (current study; Pyter et al., 2005b; Pyter et al., 2005a; Pyter et al., 2006; Workman et al., 2009). Testosterone impairs CA1 LTP in rats (Harley et al., 2000) and alters hippocampal synaptic plasticity in Mus (Sakata et al., 2000). Although species differences among rodents in the effects of testosterone on hippocampal function and LTP remain unspecified, the present study argues against testosterone impairing hippocampal LTP in white-footed mice, as the SD mice (low testosterone) reduced LTP. SD deficits in spatial learning and memory can be rescued with testosterone, however removal of testosterone from LD mice did not impair spatial learning and memory, and neither testosterone nor photoperiod altered androgen receptor expression in the hippocampus (Pyter et al., 2006). Taken together, interpretation of these results suggests that the mechanism underlying photoperiodic behavioral and physiological plasticity in the hippocampus of white-footed mice may be independent of gonadal steroids, however we cannot rule out this possibility due to potential de novo synthesis of steroids in the brain (for review see Schmidt et al., 2008).
Induction of Schaffer collateral–CA1 LTP is NMDA receptor dependent and is based upon cooperativity of AMPA and NMDA ion channels (Bliss and Collingridge, 1993). AMPA channels are responsible for early LTP (<20 min), while late-phase LTP is NMDA and AMPA channel dependent (Lisman and Raghavachari, 2006). Lower recruitment and/or availability of silent NMDA synapses or partially silent NMDA/AMPA synapses in SD may underlie the photoperiodic differences in LTP (Poncer, 2003; Lisman and Raghavachari, 2006). Additionally, AMPA-mediated transmission can be potentiated via multiple pathways by the activated alpha subunit of CaM kinase II (Lisman and Raghavachari, 2006), and melatonin impairs calmodulin and CaMKII activity (Fukunaga et al., 2002; Soto-Vega et al., 2004), which is consistent with our current findings that both early- and late-phase LTP are equally impaired in mice which had a longer duration of melatonin exposure (Figure 3). Thus, we speculate that photoperiodic differences in Ca2+ regulation, silent synapses, or expression of AMPA and NMDA receptors may underlie photoperiodic differences in LTP. However, the contribution of CA1-specific mRNA and protein expression of ion channels, proteins involved in calcium regulation, and the contribution of trophic factors such as Arc or Bdnf to SD impairment of LTP and spatial learning and memory remain to be investigated.
Male P. leucopus maintain breeding territories in the spring and early summer (King, 1968), and the observed attenuation of spatial memory and LTP may represent an adaptation to conserve energy during the short days of winter when territorial maintenance is unnecessary. In addition to energy conservation, the photoperiodic plasticity in P. leucopus described in the current and previous studies also shares some common adaptive non-reproductive photoperiodic traits with other mammals, such as alterations in immune function, affective responses, and social behaviors (reviewed in Nelson et al., 2010). The extent to which using naturalistic mammalian models of photoperiodic plasticity in brain structure and physiology, which show robust responses to a single environmental signal (viz. day length), will lead to insights into the mechanisms underlying seasonal human pathologies remain unspecified.
Acknowledgements
This work was supported by NIH grant MH57535 and NSF grant IOS-08-38098 (RJN). We thank Shannon Chen and Brittany Jones for their technical assistance, and Sally Wolfe for her expert animal care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ball GF, Balthazart J. Seasonal and hormonal modulation of neurotransmitter systems in the song control circuit. J Chem Neuroanat. 2010;39:82–95. doi: 10.1016/j.jchemneu.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Karsch FJ. Nightly duration of pineal melatonin secretion determines the reproductive response to inhibitory day length in the ewe. Biol Reprod. 1984;30:585–593. doi: 10.1095/biolreprod30.3.585. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2004;46:30–38. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Cao XJ, Wang M, Chen WH, Zhu DM, She JQ, Ruan DY. Effects of chronic administration of melatonin on spatial learning ability and long-term potentiation in lead-exposed and control rats. Biomed Environ Sci. 2009;22:70–75. doi: 10.1016/S0895-3988(09)60025-8. [DOI] [PubMed] [Google Scholar]
- Carlson LL, Zimmermann A, Lynch GR. Geographic differences for delay of sexual maturation in Peromyscus leucopus: effects of photoperiod, pinealectomy, and melatonin. Biol Reprod. 1989;41:1004–1013. doi: 10.1095/biolreprod41.6.1004. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2005;20:225–236. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DR, Davies SN. Melatonin blocks the induction of long-term potentiation in an N-methyl-D-aspartate independent manner. Brain Res. 1997;767:162–165. doi: 10.1016/s0006-8993(97)00733-6. [DOI] [PubMed] [Google Scholar]
- Delgado-Gonzalez FJ, Alonso-Fuentes A, Delgado-Fumero A, Garcia-Verdugo JM, Gonzalez-Granero S, Trujillo-Trujillo CM, Damas-Hernandez MC. Seasonal differences in ventricular proliferation of adult Gallotia galloti lizards. Brain Res. 2008;1191:39–46. doi: 10.1016/j.brainres.2007.10.092. [DOI] [PubMed] [Google Scholar]
- Dowell SF, Lynch GR. Duration of the melatonin pulse in the hypothalamus controls testicular function in pinealectomized mice (Peromyscus leucopus) Biol Reprod. 1987;36:1095–1101. doi: 10.1095/biolreprod36.5.1095. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231:491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- Feng Y, Zhang LX, Chao DM. [Role of melatonin in spatial learning and memory in rats and its mechanism] Sheng Li Xue Bao. 2002;54:65–70. [PubMed] [Google Scholar]
- Fisher LJ. Neural precursor cells: applications for the study and repair of the central nervous system. Neurobiol Dis. 1997;4:1–22. doi: 10.1006/nbdi.1997.0137. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Horikawa K, Shibata S, Takeuchi Y, Miyamoto E. Ca2+/calmodulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J Neurosci Res. 2002;70:799–807. doi: 10.1002/jnr.10400. [DOI] [PubMed] [Google Scholar]
- Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Harley CW, Malsbury CW, Squires A, Brown RA. Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus. 2000;10:693–697. doi: 10.1002/1098-1063(2000)10:6<693::AID-HIPO1007>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF. A brain for all seasons: cellular and molecular mechanisms of photoperiodic plasticity. Prog Brain Res. 2002;138:255–280. doi: 10.1016/S0079-6123(02)38082-8. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (U.S.) Guide for the care and use of laboratory animals. 7th Edition National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- King JA. Biology of Peromyscus (Rodentia) American Society of Mammalogists; [Stillwater, Okla.]: 1968. [Google Scholar]
- Lisman J, Raghavachari S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE. 2006:re11. doi: 10.1126/stke.3562006re11. 2006. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Malenka RC. The long-term potential of LTP. Nat Rev Neurosci. 2003;4:923–926. doi: 10.1038/nrn1258. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Thompson CK. Seasonal-like growth and regression of the avian song control system: neural and behavioral plasticity in adult male Gambel's white-crowned sparrows. Gen Comp Endocrinol. 2008;157:259–265. doi: 10.1016/j.ygcen.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Denlinger DL, Somers DE. Photoperiodism : the biological calendar. Oxford University Press; Oxford ; New York: 2010. [Google Scholar]
- Nguyen PV. Comparative plasticity of brain synapses in inbred mouse strains. J Exp Biol. 2006;209:2293–2303. doi: 10.1242/jeb.01985. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;1016:628–658. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- Ozcan M, Yilmaz B, Carpenter DO. Effects of melatonin on synaptic transmission and long-term potentiation in two areas of mouse hippocampus. Brain Res. 2006;1111:90–94. doi: 10.1016/j.brainres.2006.06.117. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Poncer JC. Hippocampal long term potentiation: silent synapses and beyond. J Physiol Paris. 2003;97:415–422. doi: 10.1016/j.jphysparis.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Zucker I, Nelson RJ. Seasonal rhythms of mammalian behavioral neuroendocrinology. In: Pfaff DW, editor. Hormones, brain, and behavior. Elsevier/Academic Press; Amsterdam; Boston: 2009. pp. 507–538. [Google Scholar]
- Pyter LM, Reader BF, Nelson RJ. Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus) J Neurosci. 2005a;25:4521–4526. doi: 10.1523/JNEUROSCI.0795-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Neigh GN, Nelson RJ. Social environment modulates photoperiodic immune and reproductive responses in adult male white-footed mice (Peromyscus leucopus) Am J Physiol Regul Integr Comp Physiol. 2005b;288:R891–896. doi: 10.1152/ajpregu.00680.2004. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Trainor BC, Nelson RJ. Testosterone and photoperiod interact to affect spatial learning and memory in adult male white-footed mice (Peromyscus leucopus) Eur J Neurosci. 2006;23:3056–3062. doi: 10.1111/j.1460-9568.2006.04821.x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia. 1993;49:654–664. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- Sakata K, Tokue A, Kawai N. Altered synaptic transmission in the hippocampus of the castrated male mouse is reversed by testosterone replacement. J Urol. 2000;163:1333–1338. [PubMed] [Google Scholar]
- Schmidt KL, Pradhan DS, Shah AH, Charlier TD, Chin EH, Soma KK. Neurosteroids, immunosteroids, and the Balkanization of endocrinology. Gen Comp Endocrinol. 2008;157:266–274. doi: 10.1016/j.ygcen.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Weeber EJ, Christian J, Nekrasova T, Landreth GE, Sweatt JD. A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1. Learn Mem. 2003;10:26–39. doi: 10.1101/lm.51103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Eichenbaum H. Hippocampus as a memory map: synaptic plasticity and memory encoding by hippocampal neurons. Hippocampus. 1999;9:365–384. doi: 10.1002/(SICI)1098-1063(1999)9:4<365::AID-HIPO4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Sherry DF, Hoshooley JS. Seasonal hippocampal plasticity in food-storing birds. Philos Trans R Soc Lond B Biol Sci. 2010;365:933–943. doi: 10.1098/rstb.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneaux V, Sinitskaya N, Salingre A, Garidou ML, Pevet P. Rat and Syrian hamster: two models for the regulation of AANAT gene expression. Chronobiol Int. 2006;23:351–359. doi: 10.1080/07420520500521962. [DOI] [PubMed] [Google Scholar]
- Smale L, Heideman PD, French JA. Behavioral neuroendocrinology in nontraditional species of mammals: things the ‘knockout’ mouse CAN'T tell us. Horm Behav. 2005;48:474–483. doi: 10.1016/j.yhbeh.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Vega E, Meza I, Ramirez-Rodriguez G, Benitez-King G. Melatonin stimulates calmodulin phosphorylation by protein kinase C. J Pineal Res. 2004;37:98–106. doi: 10.1111/j.1600-079X.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- Stehle JH, von Gall C, Korf HW. Organisation of the circadian system in melatonin-proficient C3H and melatonin-deficient C57BL mice: a comparative investigation. Cell Tissue Res. 2002;309:173–182. doi: 10.1007/s00441-002-0583-2. [DOI] [PubMed] [Google Scholar]
- Talaei SA, Sheibani V, Salami M. Light deprivation improves melatonin related suppression of hippocampal plasticity. Hippocampus. 2010;20:447–455. doi: 10.1002/hipo.20650. [DOI] [PubMed] [Google Scholar]
- Walton JC, Waxman B, Hoffbuhr K, Kennedy M, Beth E, Scangos J, Thompson RR. Behavioral effects of hindbrain vasotocin in goldfish are seasonally variable but not sexually dimorphic. Neuropharmacology. 2010;58:126–134. doi: 10.1016/j.neuropharm.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci. 2005;22:2231–2237. doi: 10.1111/j.1460-9568.2005.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells T, Carter DA. Genetic engineering of neural function in transgenic rodents: towards a comprehensive strategy? J Neurosci Methods. 2001;108:111–130. doi: 10.1016/s0165-0270(01)00391-0. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Workman JL, Bowers SL, Nelson RJ. Enrichment and photoperiod interact to affect spatial learning and hippocampal dendritic morphology in white-footed mice (Peromyscus leucopus) Eur J Neurosci. 2009;29:161–170. doi: 10.1111/j.1460-9568.2008.06570.x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Xiong H, Mennigen JA, Popesku JT, Marlatt VL, Martyniuk CJ, Crump K, Cossins AR, Xia X, Trudeau VL. Defining global neuroendocrine gene expression patterns associated with reproductive seasonality in fish. PLoS One. 2009;4:e5816. doi: 10.1371/journal.pone.0005816. [DOI] [PMC free article] [PubMed] [Google Scholar]