Abstract
Development of cerebellar Purkinje cells (PCs) is modulated by neuroactive steroids. Developing hippocampal pyramidal neurons retrogradely release a pregnenolone sulfate (PregS)-like neurosteroid that may contribute to glutamatergic synapse stabilization. We hypothesized that PregS could exert a similar effect on developing PCs. To test this hypothesis, we performed whole-cell patch-clamp recordings from PCs in acute cerebellar vermis slices from neonatal rats. PregS induced a robust (~3,000 %) and reversible increase in AMPA receptor-mediated miniature excitatory postsynaptic current (AMPA-mEPSC) frequency without affecting the amplitude, time-to-rise, or half-width of these events. PregS also increased the frequency of GABAA receptor-mediated miniature postsynaptic currents but to a significantly lesser extent (<100%). The PregS-induced increase of AMPA-mEPSC frequency was not significantly decreased by antagonists of receptors (NMDA, glycine, α7 nicotinic acetylcholine, and σ1) that have been shown to modulate glutamatergic transmission at PCs and/or mediate the actions of PregS on neurotransmitter release. Ca2+ chelation experiments suggested that PregS acts by increasing presynaptic terminal [Ca2+]i, an effect that is independent of voltage-gated Ca2+ channels, but is blocked by the antagonist of transient receptor potential (TRP) channels, La3+. PregS also increased the amplitude of EPSCs evoked by climbing fiber (CF) stimulation and decreased the paired-pulse ratio of these events. Neither CF- nor parallel fiber- evoked EPSCs were affected by PregS in slices from juvenile rats. These results suggest that glutamate release at CF-to-PC synapses is an important target of PregS in the neonatal cerebellar cortex, an effect that may play a role in the refinement of these synapses.
Keywords: neurosteroid, cerebellar cortex, synaptic transmission, development, Purkinje cell, climbing fiber
INTRODUCTION
The cerebellum is essential for motor coordination, motor learning, timing of conditioned reflexes, and also for a number of higher cognitive functions (Ito, 2002). Alterations in the function of neurons of the cerebellar cortex and deep cerebellar nuclei have been implicated in the pathophysiology of several neurological and psychiatric disorders, including autism, schizophrenia, alcoholism, fetal alcohol syndrome, and ataxia (Schmahmann, 2004, Ito-Ishida et al., 2008, Shi et al., 2009). The mature cerebellar cortex consists of three layers—the granule layer is the innermost layer, the Purkinje layer is the middle layer and the molecular layer is the outermost layer. The granule cell layer contains the cerebellar granule cells and Golgi cells. Cerebellar granule cells receive excitatory input from the brain stem and spinal cord via mossy fibers and inhibitory input from Golgi cells. The axons of granule cells form the parallel fibers (PFs) that provide glutamatergic input to distal dendrites of Purkinje cells (PCs). PCs also receive glutamatergic input from the inferior olive via the climbing fibers (CFs), which synapse onto more proximal dendrites. γ-aminobutyric acid (GABA)- releasing interneurons (i.e. basket and stellate cells) located in the molecular layer provide inhibitory input to PCs; these cells are collectively known as molecular layer interneurons (MLIs). PCs are GABAergic neurons that project to deep cerebellar nuclei and their axons constitute the only output of the cerebellar cortex.
PCs undergo marked morphological and functional changes during the first 2 postnatal weeks in rodents; the first 10–12 days of life in rats is equivalent to the 3rd trimester of pregnancy in humans and is a critical period for PC maturation. During this period, PCs merge into a single layer, develop an extensive dendritic tree, and form synaptic connections (Kurihara et al., 1997, Eilers et al., 2001). The development of CF-to-PC synapses precedes that of PF-to-PC synapses. CF-to-PC synapses have been detected as early as embryonic day (E) 19 (Morara et al., 2001). From the onset of CF-to-PC synaptogenesis until postnatal day (P) 4–7, the number of CF inputs per PC reaches a maximum of approximately 5. These immature CF-to-PC inputs differ in synaptic strength and decrease in number through the 3rd postnatal week by a process of synapse elimination of all but the strongest CF input (reviewed in (Bosman and Konnerth, 2009).
In immature neurons, glutamatergic and GABAergic transmission have been shown to regulate the maturation of dendrites and axons, as well as synaptogenesis and synaptic refinement. Glutamate controls dendritic arbor growth via N-methyl-D-aspartic acid (NMDA) receptor-dependent Ca2+ transients and insertion of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors (Cline and Haas, 2008). GABA acts mainly by binding to GABAA receptors, which conduct Cl− ions out of developing neurons, leading to membrane potential depolarization and activation of voltage-gated Ca2+ channels. This is in contrast to GABAA receptors in mature neurons where Cl− flows into the cell, causing membrane potential hyperpolarization (Huang et al., 2007b). Therefore, factors that modulate the glutamatergic and GABAergic neurotransmitter systems in developing neurons can have an impact on synapse formation and maturation. Among these factors are neuroactive steroids produced in the brain either from peripheral precursors or independently of these precursors (i.e. neurosteroids) (Mellon, 2007). Neurosteroids and neuroactive steroids such as allopregnanolone, progesterone, and estradiol have been shown to play a role in PC survival, dendritic growth, spine formation, and synaptogenesis (Tsutsui et al., 2003, Sasahara et al., 2007). However, it is unknown whether other neurosteroids participate in the maturation of synapses onto PCs. Work from our laboratory demonstrated that an endogenous pregnenolone sulfate (PregS)-like neurosteroid produces a long-lasting strengthening of glutamatergic transmission in developing CA1 hippocampal pyramidal neurons (Mameli et al., 2005). Based on these results, we hypothesized that PregS also enhances glutamatergic synaptic transmission at developing PC synapses. To test this hypothesis we used whole-cell patch-clamp electrophysiological techniques in acute cerebellar slices from rats and measured changes in glutamatergic transmission upon bath application of PregS. For comparison, we also tested the effect of PregS on GABAergic transmission in immature PCs.
EXPERIMENTAL PROCEDURES
Unless specified, all chemicals were from Sigma (St. Louis, MO) or Tocris Bioscience (Ellisville, MO). All experiments were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee and conformed with National Institutes of Health guidelines. Cerebellar vermis parasagittal slices (250 µm-thick) were prepared from neonatal (P4-10) or juvenile (P15-21) Sprague-Dawley rats and recordings from PCs were performed, as previously described (Mameli et al., 2008). The artificial cerebrospinal fluid (ACSF) was equilibrated with 95%O2/5%CO2 and contained the following (in mM): 126 NaCl, 2 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 2 CaCl2, 10 glucose, and 10 µM SR 95531 or 1 µM GYKI 53655 for AMPA-mEPSC and GABAA-mPSC recordings, respectively. For these recordings the membrane potential was held at −70 mV. For Ca2+ free recordings, the MgSO4 concentration was increased to 3 mM and 1 mM ethylene glycol tetraacetic acid (EGTA) was added to reduce contaminant Ca2+ levels (Perez-Velazquez et al., 1994). Patch pipette resistances were between 2 and 5.5 MΩ. AMPA-mEPSCs, GABAA-mPSCs and CF stimulation- evoked EPSCs (CF-eEPSCs) were recorded from neonatal rat slices using an internal solution containing (in mM): 150 CsCl, 1.5 MgCl2, 10 HEPES, 0.1 BAPTA (Calbiochem, La Jolla, CA), 2 Na2-ATP, 0.4 Na-GTP, at pH 7.3. For CF-eEPSC and PF-eEPSC recordings from juvenile rat slices, the internal solution contained (in mM): 120 CsOH, 120 D-gluconic acid, 3 tetraethylammonium-Cl, 10 HEPES, 0.1 BAPTA, 2 Na2-ATP, 2 Na-GTP and 0.2 Mg-ATP. The access resistance was between 10 and 30 MΩ. If the access resistance changed > 30%, the recording was discarded. Recordings of mPSCs were obtained in presence of 0.5 µM tetrodotoxin (TTX; Calbiochem, La Jolla, CA).
For CF-eEPSC recordings, an ACSF-filled glass unipolar stimulating electrode was used and was placed on the granule cell layer, ~ 150 µm from the PC. A pair of current pulses, (duration of 100 µs; intensity 20–100 µA) was delivered every 15 s with an inter-event-interval of 50 or 60 ms. The holding potential was −70 mV in neonatal rat slices. In juvenile rat slices, the amplitude of the CF-eEPSC dramatically increased with respect to that of neonatal rat slices. To improve voltage-clamp stability in juvenile rat slices, the holding potential was therefore changed to −10 mV and the Ca2+ concentration in the ACSF was lowered to 0.5 mM (Mg2+ concentration was increased to 2.5 mM). All responses evoked were typical of CF-to-PC synapses, as they were all-or-none, and showed paired-pulse depression. PF-eEPSCs were recorded from juvenile rat slices using a holding potential of −50 mV or −70 mV. For these recordings, the stimulation electrode was placed in the molecular layer (duration of 100 µs; intensity 20–100 µA). For all stimulation experiments, 4 mM QX-314 bromide was added to the internal solution. The paired-pulse ratio (PPR) was calculated as eEPSC2/eEPSC1.
Data were acquired with pClamp 9.2 or 10 (Molecular Devices, Sunnyvale, CA) at a filtering frequency of 2 kHz and an acquisition rate of 10 kHz. Miniature events were analyzed using Mini Analysis (Synaptosoft, Decatur, GA); only events that had an amplitude at least eight times greater than the noise (average 0.84 ± 0.04 pA; n = 11) were selected for analysis. Individual recordings were analyzed using the Kolmogorov-Smirnov (K-S) test using a conservative value for significance of p < 0.01. Evoked EPSCs were analyzed with Clampex 9.2 (Molecular Devices, Sunnyvale, CA). Statistical analyses of pooled data were performed with GraphPad Prizm 4 (GraphPad Software, San Diego, CA). Data were initially analyzed with the D’Agostino and Pearson omnibus normality test. If data followed a normal distribution, these were analyzed using parametric tests. If this was not the case, then non-parametric tests were used. The level of significance was p < 0.05. Data were normalized to baseline and expressed as percent of baseline, or as change in Hz ± SEM.
RESULTS
PregS enhances the frequency but not the amplitude of AMPA-mEPSCs in neonatal PCs
We tested the effect of PregS on AMPA-mEPSCs in neonatal rat (P4-10) slices. These events were recorded in presence of the GABAA receptor antagonist, SR 95531 (10 µM) and, under these conditions, were completely blocked by the AMPA receptor antagonist, GYKI 53655 (50 µM; n = 9; not shown). Fig 1A–C shows that bath application of PregS (25 µM) for 5 min, reversibly enhanced AMPA-mEPSC frequency without having an effect on amplitude. The effect of PregS on AMPA-mEPSC frequency was robust (3,580 ± 1,073 % of baseline; p < 0.0001, n=21) (Fig 1C). K-S test analysis revealed that the AMPA-mEPSC inter-event interval was significantly decreased in 21 out of 21 neurons, whereas the amplitude was increased in 5 out of 21 neurons, decreased in 6 out of 21 and was not affected in 10 out of 21. The frequency of AMPA-mEPSCs (in Hz) in the absence and presence of PregS is shown in Fig 1C and Table 1; PregS increased the frequency of these events approximately 13-fold. The percent increase in the frequency of these events was inversely correlated with the baseline frequency, while the increase in Hz showed no correlation with the baseline frequency (Fig 1D). PregS did not affect the AMPA-mEPSC time-to-rise (107 ± 4.6 % of baseline) or half-width (102 ± 2.8 % of baseline) (not significant by Wilcoxon Signed Rank Test; n = 21) (Table 1). We also tested the effect of a lower concentration of PregS (5 µM) on the frequency of AMPA-mEPSCs and found that it significantly enhanced AMPA-mEPSC frequency (401.1 ± 114.7 % of baseline; raw change from baseline 0.6 ± 0.26 Hz; n=4; the K-S test showed a significant reduction in the inter-event interval in 3 out of 4 cells) without affecting amplitude, time-to-rise or half-width (data not shown). To determine whether PregS had an effect on AMPA-mEPSC frequency in slices from older animals, we measured its effect at P12. Using a Cs-gluconate-based solution, we found that 25 µM PregS significantly increased AMPA-mEPSC frequency but to a lesser extent than in PCs from P4-10 rats (184.3 ± 13.83 % of baseline; raw frequency change 0.97 ± 0.46 Hz; n=4; KS test <0.01 in all 4 cells).
Figure 1.
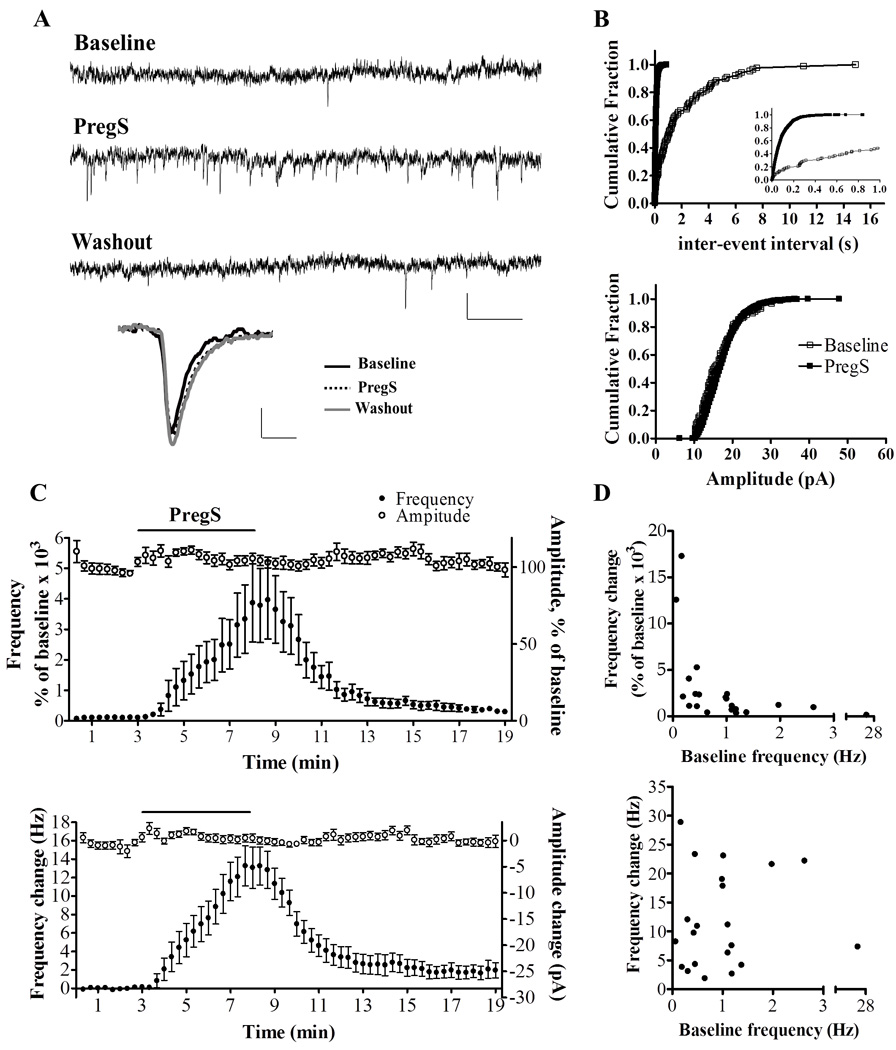
PregS (25 µM) robustly increases the frequency of AMPA-mEPSCs in PCs from neonatal rats. (A) Sample traces for a P5 rat, calibration: 20 pA, 0.2 s. Low panel average events, calibration: 4.1 pA, 3 ms. (B) Corresponding cumulative probability plots; PregS decreased the inter-event interval (p < 0.01 by K–S test; 86 events/3 min before PregS and 2050 events/3 min during PregS); the inset further illustrates the robust increase that PregS induces on the inter-event interval at an expanded scale. The bottom panel shows that amplitude of these events is not significantly affected by PregS. (C) Average time courses of AMPA-mEPSC frequency and amplitude expressed as a percent of baseline (top panel) and as the raw change (bottom panel) (n=21). (D) The percent increase in frequency induced by PregS is inversely correlated to the baseline frequency (r = −0.7390; p < 0.05 by Spearman correlation test, n=21) (top panel). However, the PregS-induced increase in frequency in Hz is not correlated to the baseline frequency (r=−0.09775; p=0.6734 by Pearson correlation test) (bottom panel).
Table 1.
AMPA-mEPSC parameters in PCs from neonatal rats in the absence and presence of PregS or pharmacological receptor blockers.
| Experimental condition (n) |
Frequency (Hz) |
Amplitude (pA) |
Time-to-rise (ms) |
Half-Width (ms) |
|---|---|---|---|---|
| Control (11) | 0.80 ± 0.22 | 23.20 ± 1.55 | 2.27 ± 0.12 | 1.98 ± 0.12 |
| APV + Strychnine (11) | 0.81 ± 0.16 | 24.71 ± 1.68 | 2.93 ± 0.39 | 2.23 ± 0.14 |
| BD1063 (9) | 0.49 ± 0.14 | 20.16 ± 1.19 | 2.54 ± 0.25 | 2.18 ± 0.13 |
| MLA (10) | 0.31 ± 0.10 | 23.44 ± 2.72 | 2.69 ± 0.28 | 2.13 ± 0.24 |
| BAPTA-AM (9) | 0.66 ± 0.31 | 21.36 ± 1.46 | 2.79 ± 0.42 | 2.56 ± 0.3 |
| PregS (11) | 12.2 ± 1.98 *** | 22.62 ± 1.50 | 2.59 ± 0.20 | 2.07 ± 0.15 |
| Cd2+ (11) | 1.79 ± 0.58 | 20.75 ± 1.52 | 2.08 ± 0.20 | 2.08 ± 0.16 |
| La3+ (11) | 0.44 ± 0.17 | 23.89 ± 2.69 | 2.05 ± 0.12 | 1.97 ± 0.15 |
p<0.01 vs. control by one-way ANOVA followed by Dunn’s multiple comparison test.
PregS enhances AMPA-mEPSC frequency independently of neurotransmitter receptors
PregS has been shown to modulate glutamate release onto immature as well as mature neurons via modulation of a number of neurotransmitter receptors; namely, NMDA, σ1 and α7 nicotinic acetylcholine receptors (α7nACh) (Meyer et al., 2002, Chen and Sokabe, 2005, Mameli et al., 2005, Schiess and Partridge, 2005). In addition, activation of NMDA and glycine receptors has been shown to facilitate transmitter release onto developing PCs (Kawa, 2003, Bidoret et al., 2009). Previous findings in the developing hippocampus suggested that PregS enhances glutamate release onto CA1 pyramidal neurons from neonatal rats via activation of presynaptic NMDA receptors (Mameli et al., 2005). In cultured hippocampal neurons PregS enhances mEPSC frequency via modulation σ1 receptors, and at perforant path-to-dentate granule cell synapses, it produces a similar effect via modulation of α7nACh receptors (Kawa, 2002, Meyer et al., 2002, Chen and Sokabe, 2005). Therefore, we measured the effect of PregS on AMPA-mEPSCs in the presence of DL-2-Amino-5-phosphonopentanoic acid (DL-APV) (100 µM), strychnine (1 µM), BD-1063 (1 µM), and methyllycaconitine (MLA; 200 nM) to block NMDA, glycine, σ1, and α7nACh receptors, respectively. In presence of these agents, PregS increased AMPA-mEPSC frequency to a similar extent as under control conditions (Fig 2). The K-S test showed that PregS significantly decreased the inter-event interval of AMPA-mEPSCs in all cells regardless of the pharmacological blocker used. Basal AMPA-mEPSC properties were not significantly affected by any of these agents (Table 1).
Figure 2.
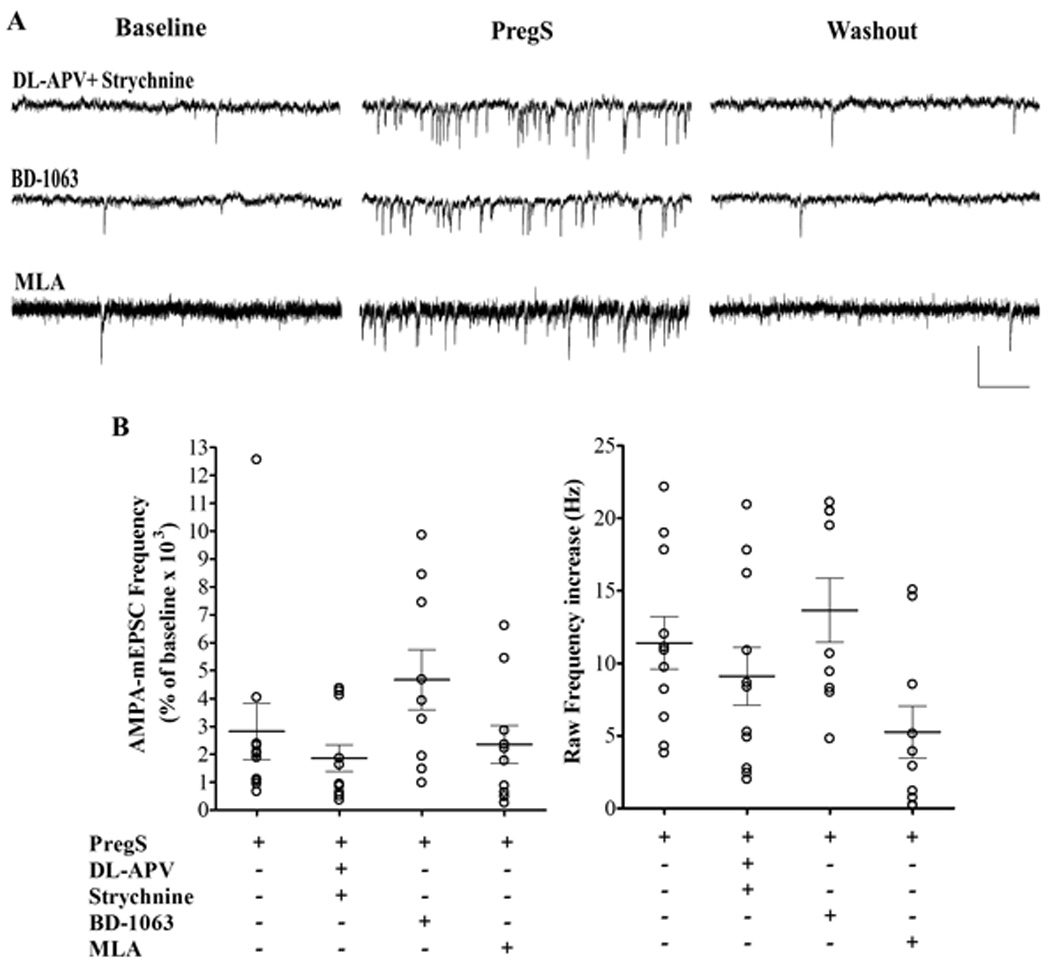
The PregS (25 µM)-induced increase in AMPA-mEPSC frequency is independent of NMDA, glycine, σ1, or α7nACh receptors. (A) Sample traces illustrating the PregS effect on AMPA-mEPSC frequency in PCs from P4-5 rats in the presence of the NMDA and glycine receptors blockers, DL-AP5 (100 µM) and strychnine (1 µM); the σ1 receptor blocker, BD-1063 (1 µM); and the α7nACh receptor blocker, MLA (200nM); calibration: 50 pA and 0.2 s. (B) Summary graphs of the percent frequency (left panel) and the raw frequency increases (right panel) induced by PregS in the presence of NMDA, glycine, σ1 or α7nACh receptor antagonists. For both, percent and raw frequency increases, one-way ANOVA followed by Dunn's multiple comparison test showed no significant difference between controls (n=11) and DL-APV/strychnine (n=11), BD-1063 (n=9) or MLA (n=10). The effect of PregS on AMPA-mEPSC frequency from Fig 1 is shown again for comparison.
PregS enhances AMPA-mEPSC frequency by increasing presynaptic [Ca2+]i via activation of a TRP channel
We next used the membrane-permeable Ca2+ chelator, BAPTA-AM (50 µM), to test the role of Ca2+ in the mechanism of action of PregS. A 10 minute incubation with BAPTA-AM, followed by a 5 minute washout, resulted in no significant change in basal AMPA-mEPSC frequency (Table 1) and a significant reduction of the PregS-induced increase of AMPA-mEPSC frequency (565.2 ± 379 % of baseline, **p < 0.05; raw frequency change of 0.65 ± 0.3 Hz from baseline, ***p <0.05, Fig 3) when compared to controls (2,824 ± 1,014 % of baseline; raw frequency change 11.41 ± 1.82 Hz from baseline, Fig 3B). It is noteworthy that, with this treatment, the K-S test showed that, 5 out of 9 cells showed no significant change in the AMPA-mEPSC inter-event interval with PregS bath application, and the remaining 4 showed a significant decrease. BAPTA-AM is expected to non-selectively chelate Ca2+ both in the pre- and post-synaptic compartments. To determine whether PregS acts by selectively increasing Ca2+ in one of these compartments, we tested the effect of postsynaptic Ca2+ chelation by dialyzing BAPTA (10 mM) into PCs through the patch pipette. K-S test showed that in 3 out of 3 cells, PregS significantly enhanced the frequency of AMPA-mEPSCs (2,541 ± 864.4 % of baseline; raw frequency increase of 13.69 ± 7.15 Hz from baseline, n=3, data not shown), suggesting that PregS acts by increasing [Ca2+]i in presynaptic terminals.
Figure 3.
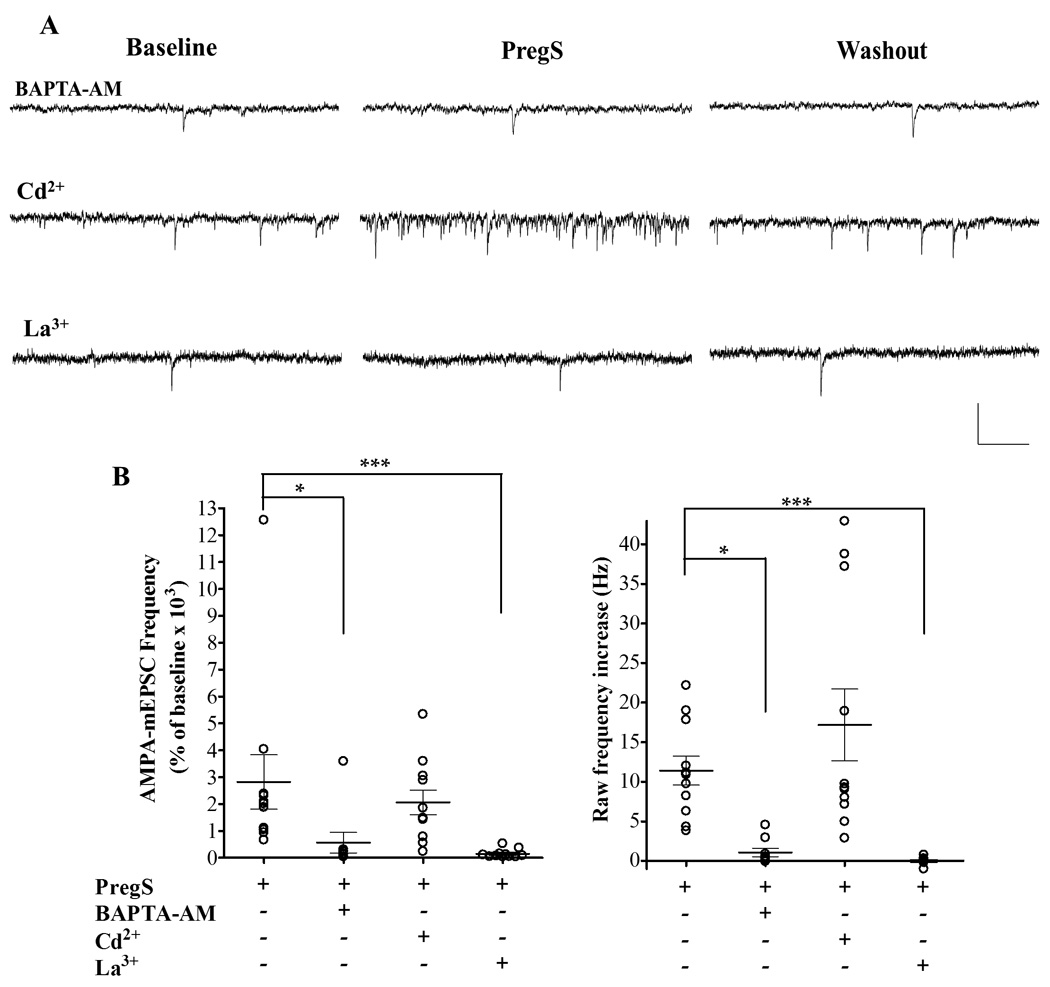
PregS (25 µM) increases AMPA-mEPSC frequency by elevating [Ca2+]i possibly via modulation of TRP channels. (A) Sample traces illustrating the effect of PregS on AMPA-mEPSC frequency in PCs from P4-5 rats in the presence of BAPTA-AM, Cd2+ and La3+, calibration: 50 pA and 0.2 s. (B) Summary graphs of the percent frequency (left panel) and the raw frequency (right panel) changes induced by PregS in the presence of these agents. For both, percent and raw frequency increases, one-way ANOVA followed by Dunn's multiple comparison test showed a significant difference between controls (n=11), BAPTA-AM (n=9; * p < 0.05) and La3+ (n=11; *** p < 0.001). The effect of PregS on AMPA-mEPSC frequency from Fig 1 is shown again for comparison.
To further assess if Ca2+ entrance into the presynaptic terminal was required for this effect of PregS, we performed experiments in Ca2+ free ACSF in the presence of the Ca2+ chelator, EGTA (1mM); under these conditions, the baseline AMPA-mPSC frequency (0.25 ± 0.11 Hz) was not significantly different from control (p> 0.05 by unpaired t-test, n=4; see Table 1 for control values) and the K-S test showed that in 4 out of 4 cells PregS did not significantly change the AMPA-mEPSC inter-event interval (132.6 ± 28.01 % of baseline, not significant by Wilcoxon Signed Rank Test; raw frequency change of 0.027 ± 0.043 Hz from baseline, n=4, data not shown).
At calyx of Held developing synapses, PregS was shown to increase glutamate release via facilitation of voltage-gated Ca2+ channels (VGCCs) (Hige et al., 2006). To test the involvement of these channels in the effect of PregS on neonatal PCs, we performed recordings in the presence of Cd2+ (100 µM), a broad spectrum VGCC antagonist. As shown in Table 1, Cd2+ did not significantly change basal AMPA-mEPSC parameters. Moreover, in the presence of Cd2+, PregS potently increased the frequency of AMPA-mPSCs (2,064 ± 458.30 % of baseline with a raw frequency increase of 17.18 ± 4.53 Hz, n= 11, Fig 3), suggesting that VGCC are not involved in this effect.
Recently, transient receptor potential (TRP) cation channels have been shown to be modulated by PregS, which results in increases in [Ca2+]i (Wagner et al., 2008, Lee et al., 2010). Therefore, we tested the effect of La3+, a blocker of a number of TRP channel subtypes (Albert et al., 2006). A 30 min incubation with La3+ (100 µM) did not significantly decrease baseline AMPA-mEPSC frequency (Table 1). Taken together with the lack of an effect of BAPTA-AM and Ca2+-free ACSF on basal mEPSC frequency, this result suggests that if tonic levels of PregS are present at CF-to-PC synapses, these are not sufficiently high to support quantal glutamate release, in agreement with our previous finding that a significant level of neuronal activity is required for retrograde release of endogenous PregS-like neurosteroids (Mameli et al., 2005). However, after incubation with La3+, PregS did not significantly increase AMPA-mEPSC frequency (152.2 ± 48.07 % of baseline; raw frequency change of −0.0021 ± 0.13 Hz, n= 11, Fig 3), suggesting that PregS increases AMPA-mEPSC frequency via modulation of a TRP channel.
PregS increases PPR at CF-to-PC synapses in neonatal rat slices but does not affect PPR in CF- and PF-to-PC synapses in juvenile rat slices
During the neonatal period in rats, CFs are the major glutamatergic input to PCs, with PF connections beginning to arrive after the first postnatal week (Bosman and Konnerth, 2009, Cesa and Strata, 2009). To determine whether PregS enhanced AMPA-mEPSC frequency by potentiating glutamate release at CF-to-PC synapses, we measured paired-pulse plasticity of CF-eEPSCs in neonatal PCs. These evoked responses were abolished in the presence of the AMPA receptor blocker, GYKI 53655 (50 µM). We found that CF-eEPSC1 amplitude was significantly increased to 135.5 ± 2.43 % of control, from a baseline value of 1,262 ± 258.8 pA (p < 0.001 vs 100%; n=10) in the presence of PregS (25 µM) and this effect was partially reversible (Fig 4). Increases in the probability of transmitter release are typically associated with decreases in the PPR. We found that PregS induced a significant and reversible decrease in the PPR to 75.87 ± 2.31 % of control, from a baseline value of 0.39 ± 0.03 (p < 0.001 vs 100%; n = 10). This finding suggests that PregS increases glutamate release probability at CF-to-PC synapses.
Figure 4.
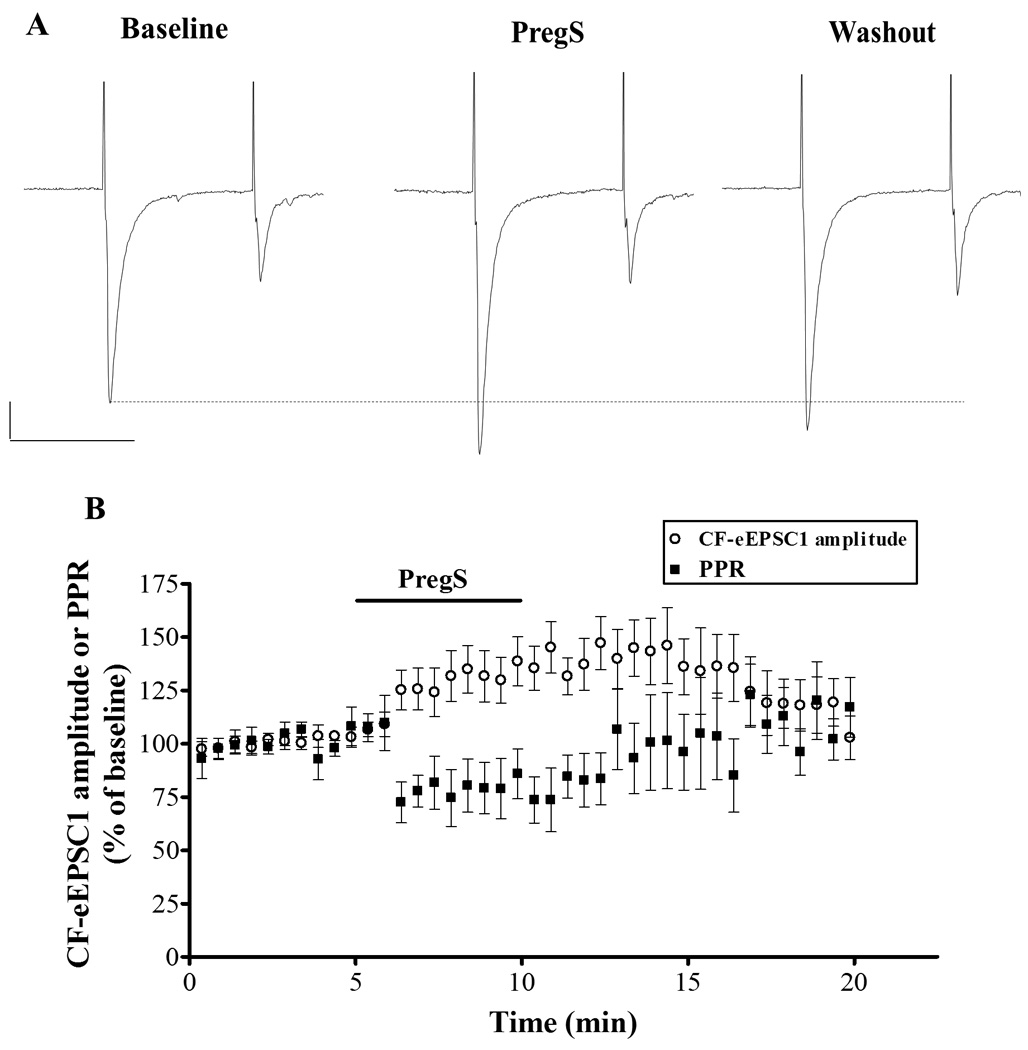
PregS (25 µM) increases CF-eEPSC amplitude and decreases the PPR in PCs from neonatal rats. (A) Sample traces from a P6 rat, calibration: 500 pA, 50 ms. (B) Average time course of the effect of PregS (25 µM) on the amplitude on CF-eEPSC1 and PPR (n=10).
CF mono-innervation is achieved during the first three postnatal weeks, during which CF-to-PC synapses undergo three distinct developmental phases. During the first postnatal week, multinumerary CF synapses onto one PC, which are composed of a strong CF synapse and several weaker ones, become functionally differentiated. This process is thought to be achieved via competition among CF inputs and is followed by an early phase and a late phase of synapse elimination of redundant inputs, the last one being dependent on PF-to-PC synapse formation (Kano and Hashimoto, 2009). We next investigated whether PregS also enhanced glutamate release from CFs beyond the functional differentiation phase (i.e. during the first week) and the early phase of synapse elimination (i.e. during P7-12). To this end, we performed paired-pulse stimulation recordings of CF-eEPSCs in juvenile rat slices (P15-21) and found that PregS (25 µM) did not significantly affect the PPR of these events (baseline PPR was 0.42 ± 0.04; Fig 5). The amplitude of CF-eEPSC1 also remained unchanged in the presence of PregS. These results suggest that PregS increases glutamate release probability at the CF-to-PC synapse only during the phases of functional differentiation and early synapse elimination.
Figure 5.
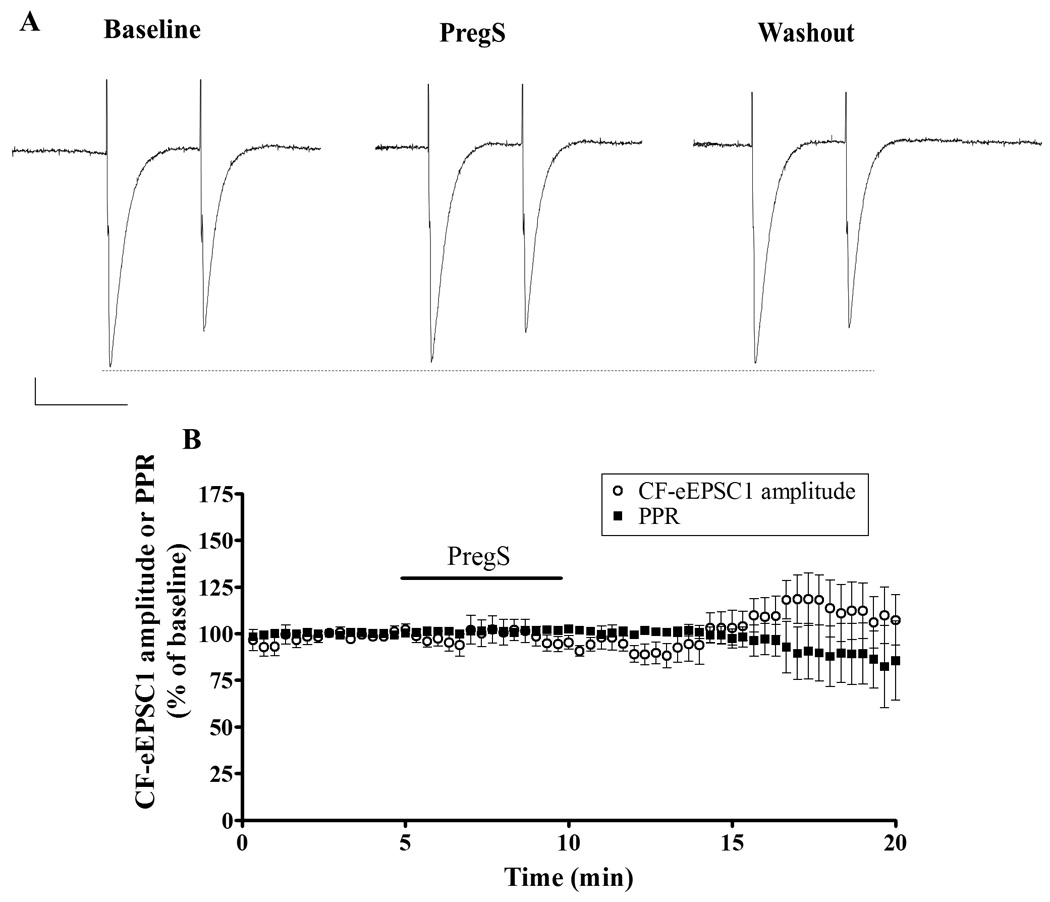
PregS (25 µM) does not affect CF-eEPSC amplitude or the PPR in PCs from juvenile rats. (A) Sample traces from a P15 rat, calibration: 100 pA, 50 ms. (B) Average time course of CF-eEPSC1 amplitude and PPR (n=7).
We also tested whether PregS affected glutamate release at PF-to-PC synapses, which start forming at the end of the first postnatal week, continuing their development through the end of the third postnatal week when the migration of granule cells is completed and the outer granule cell layer disappears (Kurihara et al., 1997, Scelfo and Strata, 2005). To this end, we recorded paired-pulse plasticity of PF-eEPSCs in juvenile rat slices. We found that PregS did not change the amplitude or the PPR of PF-eEPSCs (Fig 6), suggesting that the effect of PregS on glutamate release is specific to developing CF inputs.
Figure 6.
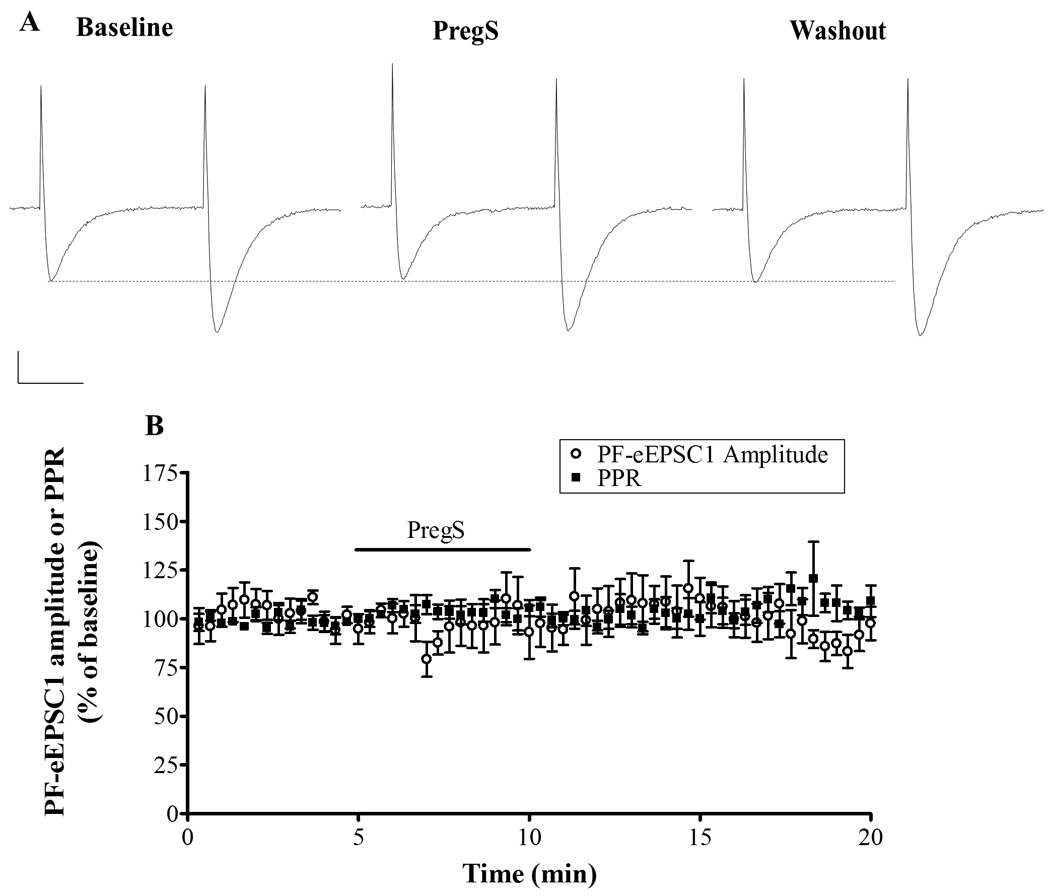
PregS (25µM) does not affect PF-eEPSC amplitude or the PPR in PCs from juvenile rats. (A) Sample traces from a P18 rat, calibration: 200 pA, 20 ms. (B) Average time course of PF-eEPSC1 amplitude and PPR (n=6).
PregS also increases GABA release in developing PCs but to a lesser extent than glutamate release
For comparison, we investigated the effect of PregS on GABAergic transmission at PCs. PregS has been shown to decrease the frequency of spontaneous inhibitory PSCs (IPSCs) as well as mIPSCs in cultured pyramidal hippocampal neurons (Teschemacher et al., 1997, Mtchedlishvili and Kapur, 2003), but it has been suggested to increase spontaneous IPSCs in PCs (Tsutsui and Ukena, 1999). We found that PregS (25 µM) also enhanced the frequency of GABAA-mPSCs in neonatal rat slices (183.9 ± 28.37 % of baseline, with a raw frequency increase of 0.84 ± 0.33 Hz from baseline, n=10; p < 0.001 by one sample t-test; the K-S test revealed that 6 out of 10 cells showed an statistically significant decrease in GABAA-mPSC inter-event interval) (Fig 7; Table 2), . In addition, PregS significantly decreased the amplitude of these events (80.88 ± 5.35 % of baseline, p < 0.05 by one sample t test; raw amplitude decrease 18.52 ± 6.17 pA from baseline) (Table 2). Time-to-rise (99.86 ± 2.98 % of baseline), and half-width (97.66 ± 2.69 % of baseline) were not significantly affected by PregS application (Table 2).
Figure 7.
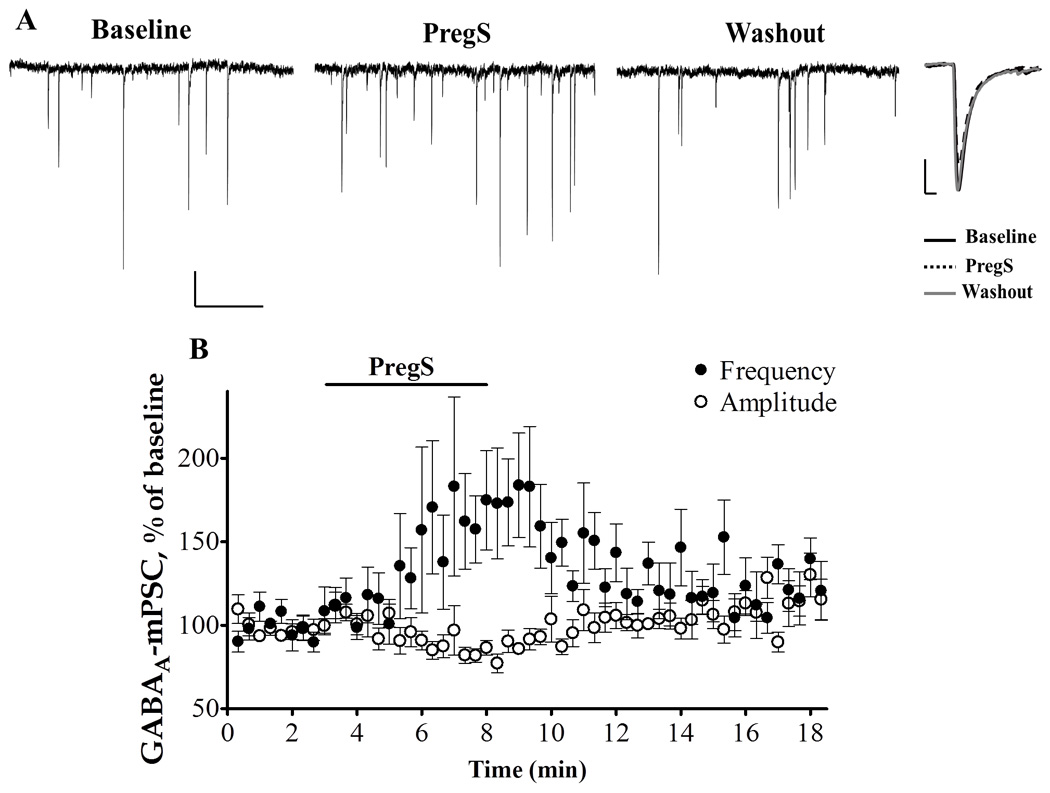
PregS (25µM) increases the frequency of GABAA-mPSCs in PCs from neonatal rats (A) representative traces from a P6 rat. Calibration: 50 pA, 1 s. Right panel average events, calibration: 20.5 pA, 4 ms. (B) Time courses of the PregS- induced changes of GABAA-mPSC frequency and amplitude.
Table 2.
GABAA-mPSC parameters in PCs from neonatal rats in the absence and presence of PregS (25 µM).
| Experimental condition (n=10) |
Frequency (Hz) |
Amplitude (pA) |
Time-to-rise (ms) |
Half-Width (ms) |
|---|---|---|---|---|
| Control | 1.88 ± 0.90 | 85.77 ± 10.35 | 2.79 ± 0.08 | 4.64 ± 0.23 |
| PregS | 2.72 ± 1.14 ** | 67.25 ± 7.54 * | 2.78 ± 0.10 | 4.47 ± 0.20 |
p < 0.05 by paired t-test,
p < 0.05 by Wilcoxon signed rank test.
DISCUSSION
PregS enhances quantal glutamate release onto neonatal cerebellar PCs
A miniature postsynaptic current is produced by the opening of postsynaptic neurotransmitter receptors in response to the presynaptic release of individual quanta of neurotransmitter. Therefore, increases in the frequency of these events, are indicative of an increase in presynaptic neurotransmitter release. On the other hand, changes in the amplitude, time-to-rise, or half-width of miniature postsynaptic currents suggest an alteration in the current through postsynaptic neurotransmitter receptors and are, therefore, indicative of a postsynaptic change. We report here that, in neonatal PCs, PregS increases the frequency of AMPA-mPSCs, but does not affect the amplitude, time–to-rise, or half-width of these events, indicating that it acts presynaptically by increasing the release of glutamate onto PCs. This effect was observed at concentrations of PregS (5 and 25 µM) that have been previously shown to increase quantal glutamate release and are near the estimated concentration of an endogenous PregS-like neurosteroid in glutamatergic synapses of developing CA1 pyramidal neurons (i.e., 17 µM); (Meyer et al., 2002, Dong et al., 2005, Mameli et al., 2005, Hige et al., 2006, Lee et al., 2010). To the best of our knowledge, the PregS effect at PC glutamatergic transmission shown here is the most robust action of this agent on quantal transmitter release reported to-date. Previous studies have shown that 25 µM PregS increases mEPSC frequency in developing hippocampal synapses by about 400% (Mameli et al., 2005), and in the mature prelimbic cortex, a similar concentration of PregS (20 µM) enhanced mEPSC frequency by about 200% (Dong et al., 2005). Moreover, the effect of PregS on glutamatergic transmission in PCs was reversible, in contrast to its effect on immature CA1 pyramidal neurons, suggesting that the late postsynaptic phase of the PregS-induced plasticity—that involves NMDA receptor-dependent recruitment of AMPA receptors into silent syapses in CA1 pyramidal neurons— does not take place in PCs (Mameli et al., 2005). A possible explanation for this finding is that postsynaptic NMDA receptors in developing PCs have different characteristics which make them unable to recruit AMPA receptors into the membrane in response to changes in glutamate release.
The PregS-induced increase of quantal glutamate release onto PCs is independent of activation of neurotransmitter receptors
Another unique property of the effect of PregS at PCs is that it was insensitive to blockade of a number of receptors (NMDA, glycine, α7nACh and σ1 receptors), which have been previously shown to mediate PregS effects and/or modulate neurotransmitter release onto neonatal PCs. Although activation of NMDA receptors mediates the PregS-induced increase in glutamate release in neonatal hippocampal neurons (Mameli et al., 2005), this is not the case in the neonatal cerebellum. Postsynaptic NMDA receptors have been shown to contribute to CF-EPSCs before P21 (Piochon et al., 2007); however, presynaptic NMDA receptors may not be present at neonatal CF-to-PC synapses and this could be an explanation for their lack of contribution to the mechanism of action of PregS. An alternative target of PregS that we considered was the glycine receptor, which has been shown to enhance the frequency of spontaneous EPSCs and IPSCs in neonatal PCs (Kawa, 2003). Nonetheless, in the presence of strychnine, the PregS-induced increase in AMPA-mEPSC frequency was not affected. These results are not surprising given that the glycine-induced increase in sEPSC frequency was almost completely abolished by TTX (Kawa, 2003), and that PregS has been shown to inhibit recombinant glycine receptors (reviewed in (Gibbs et al., 2006). Another plausible candidate for the effect of PregS on PCs was the Ca2+ permeable α7nACh receptor. This receptor has been shown to modulate the PregS-induced facilitation in synaptic transmission in the hippocampus (Chen and Sokabe, 2005). In addition, acetylcholine increases spontaneous EPSC and IPSC frequency in developing PCs (Kawa, 2002). We found that blockade of α7nACh receptor did not affect the PregS-induced increase in AMPA-mEPSC frequency. PregS has also been shown to modulate glutamate release via activation of metabotropic σ1 receptors. PregS-induced modulation of σ1 receptors enhances facilitated glutamate release in the mature hippocampus (Schiess and Partridge, 2005), and increases spontaneous glutamate release in cultured hippocampal neurons (Meyer et al., 2002). We found that blockade of σ1 receptors did not abolish the effect of PregS in PCs. Moreover, the σ1 receptor agonist, pre-084 did not affect AMPA-mEPSC frequency in P9-12 PCs (n=4, data not shown), suggesting that these receptors do not play a role in the spontaneous release of glutamate onto neonatal PCs. Taken together, these findings indicate that NMDA, glycine, α7nACh and σ1 receptors are not involved in the mechanism of action PregS at neonatal PCs.
The PregS-induced increase of quantal glutamate release requires an elevation in presynaptic [Ca2+]i and is dependent on activation of TRP channels
Previously, PregS-induced increases in glutamate release have been shown to be dependent on elevations in [Ca2+]i; incubation with the membrane permeable Ca2+ chelator, BAPTA-AM, blocked the PregS-induced increase of AMPA-mEPSC frequency in developing hippocampal neurons (Meyer et al., 2002, Mameli et al., 2005). Consistent with these studies, we found that pre-incubation with BAPTA-AM (50 µM), followed by a 5 minute washout of BAPTA-AM before PregS bath application, dramatically decreased the PregS-induced increase of AMPA-mEPSC frequency. However, this treatment did not completely abolish the effect of PregS on neonatal PCs, which could be explained by incomplete Ca2+ chelation. Absence of extracellular Ca2+ abolished the PregS-induced increase in AMPA-mEPSC frequency in neonatal PCs. This result, combined with the fact that dyalizing BAPTA into the PCs through the patch pipette did not block the increase in mEPSC frequency, supports the conclusion that elevation in Ca2+ entrance into the presynaptic terminal is required for this effect of PregS.
We found that the PregS-induced increase in AMPA-mEPSC frequency is blocked by La3+. This finding is in agreement with the results of a recent study showing that PregS increases sEPSC frequency in dentate gyrus hilar neurons, an effect that was blocked by non-selective TRP channels antagonists, including La3+ (Lee et al., 2010). La3+ antagonizes several members of the canonical (C) family of TRP channels (TRPC3, 5, 6, and 7) (Albert et al., 2006), suggesting that PregS could act by potentiating these channels. TRPC channels have been found to be expressed in presynaptic nerve terminals (Goel et al., 2002, Nichols et al., 2007) and their activation has been implicated in neurotransmitter release modulation (Selvaraj et al.). Interestingly, in the cerebellum, the expression of TRPC channels has been shown to be developmentally regulated; during the first 6 postnatal weeks, TRPC3 is abundantly expressed in comparison to TRPC4 and TRPC6 channels (Huang et al., 2007a). Additionally, TRPC3 channels mediate mGluR1-dependent slow EPSCs in PCs from young mice whereas TRPC1 channels mediate these currents in PCs from older animals (Kim et al., 2003, Hartmann et al., 2008). Given that the effect of PregS on glutamate release at PCs is restricted to the neonatal period, it is possible that the loss of PregS sensitivity in more mature neurons is a consequence of a change in the subunit composition of cerebellar TRPC channels.
An alternative TRP channel that could mediate the effects of PregS is TRPM3 (Kraft and Harteneck, 2005). PregS was shown to activate both recombinant TRPM3 channels expressed in HEK 293 cells and also native TRPM3 channels expressed in pancreatic β cells and vascular smooth muscle, where PregS stimulates insulin secretion and contraction, respectively (Wagner et al., 2008, Naylor et al., 2010). La3+ has been shown to antagonize recombinant TRPM3 channels expressed in HEK 293 cells (Grimm et al., 2003). Importantly, TRPM3 channels were recently found to be expressed in developing neurons of the cerebellum and brain stem of Wistar rats — interestingly, as development progresses, expression of these channels becomes restricted to oligodendrocytes (Hoffmann et al., 2010) and this could explain the lack of PregS sensitivity of PCs in slices from juveniles rats. Clearly, the role of TRPM3 channels in the mechanism of action of PregS in the developing cerebellum should be further investigated.
PregS enhances glutamate release evoked by electrical stimulation of CFs in slices from neonatal rats
The all-or-none responses produced by CFs on mature PCs are mediated by a massive release of glutamate that nearly saturates the postsynaptic AMPA receptors (Konnerth et al., 1990). Therefore, at these synapses, an increase in glutamate release probability would not be expected to dramatically increase CF AMPA-eEPSC amplitude (Ohtsuki and Hirano, 2008). Accordingly, the effect of PregS on AMPA-eEPSCs was substantially smaller than on AMPA-mEPSCs. Interestingly, we found that the effect of PregS on the probability of glutamate release at PCs is specific to CF synapses during the neonatal period (i.e. P4-10), where CF-to-PC synapses undergo differentiation and the first stages of elimination; importantly, PregS did not significantly affect glutamatergic transmission at CF-to-PC synapses (and also PF-to-PC synapses) beyond these developmental period (i.e. in slices from P15-21 rats). Thus, during this restricted developmental period, PregS could act on CF-to-PC synapses to strengthen glutamatergic transmission. Studies from our laboratory have shown that a PregS-like neurosteroid can be retrogradely released upon depolarization of hippocampal CA1 pyramidal cells, which subsequently increases glutamate release onto these cells (Mameli et al., 2005). In a similar fashion, CF-induced depolarization of neonatal PCs could stimulate the retrograde diffusion of a PregS-like neurosteroid, which in turn would increase the probability of glutamate release at CF terminals via activation of TRP channels, thereby contributing to the maturation of these synapses. It is important to note that the CF-eEPSC evaluated in this study were the largest ones found in each cell, representing the responses of both weak and strong CF inputs. Previous studies have shown that strong and weak CF synapses differ in their response to stimulation; while strong CF synapses undergo long term potentiation, under the same stimulation paradigm, weak CF synapses undergo long term depression (Bosman et al., 2008, Ohtsuki and Hirano, 2008). Therefore, future studies should investigate whether PregS differentially modulates the probability of glutamate release and plasticity in strong versus weak CF-to-PC synapses and how this modulation affects CF synaptic competition and the subsequent achievement of monoinnervation.
PregS increases GABAergic transmission onto neonatal PCs
We found that PregS significantly enhanced the frequency and decreased the amplitude of GABAA-mPSCs. Our findings are in contrast with previous results showing that PregS decreases GABAA-mPSC frequency in hippocampal neurons, suggesting a decrease in GABA release upon PregS application (Teschemacher et al., 1997, Mtchedlishvili and Kapur, 2003). Both of these studies were performed in cultured hippocampal neurons (3–9 week in vitro) that were obtained from late embryonic or neonatal rats, where it was found that PregS reduced the frequency of mIPSCs by 20 to 40% at concentrations between 30 nM and 50 µM (Teschemacher et al., 1997, Mtchedlishvili and Kapur, 2003). The discrepancy between the previous studies and our current findings could be due to differences in presynaptic release mechanisms, and their sensitivity to PregS, in hippocampal neurons vs. PCs. During the first postnatal week, GABAA receptors are excitatory and have been suggested to contribute to the activity-dependent maturation of cerebellar synapses, including those of CFs (Eilers et al., 2001, Watt et al., 2009). Therefore, the PregS-induced enhancement of GABAergic transmission during this developmental period could play a role in the maturation of GABAergic as well as glutamatergic synapses onto PCs. However, the specific source for the PregS-sensitive GABA release sites remains undetermined, as molecular layer interneurons to PC synapses start developing at the end of the first postnatal week (Ango et al., 2004). A plausible candidate are the PC axonal collaterals to neighboring PCs, which have been detected as early as P4 (Watt et al., 2009).
The PregS-induced decrease in the amplitude of GABAA-mPSCs is in agreement with previous reports showing that PregS inhibits GABAA receptor-mediated currents (Akk et al., 2001). However, Mtchedlishvili and Kapur, (2003) found no effect of PregS on the amplitude of GABAA mediated events and Teschemacher et al., (1997) reported a non-reversible 15% decrease in the amplitude of mIPSCs upon PregS application. This effect, however, was not attributable to a PregS- induced decrease in GABAA receptor mediated currents, because of its late onset and lack of concentration dependency, and was better explained by current run-down. Our finding that the effect of PregS on GABAA-mPSC amplitude is reversible (see Fig. 7B) suggests that PregS could directly inhibit postsynaptic GABAA receptors.
Conclusion
Cerebellar PCs are highly neurosteroidogenic cells. The cytochrome P450scc, which catalyzes the conversion of cholesterol to the neurosteroid precursor pregnenolone, has been found in the soma and dendrites of PCs in rodents, avians, amphibians and other lower vertebrates. This enzyme appears in rat PCs immediately after their differentiation (reviewed in (Tsutsui and Ukena, 1999). Therefore, it is possible that PCs synthesize an endogenous counterpart of PregS, which could play a role in the maturation of CF-to-PC synapses (for a detailed discussion on whether PregS per se is a veritable neurosteroid, see (Dong et al., 2005, Schumacher et al., 2008, Valenzuela et al., 2008). Importantly, PCs are highly sensitive to developmental ethanol exposure (Servais et al., 2007) and studies from our laboratory have shown that chronic prenatal ethanol exposure increases the levels of PregS-like neurosteroids in the developing brain and that ethanol increases the efficacy of immature hippocampal synapses in a PregS-like neurosteroid-dependent manner (Caldeira et al., 2004, Mameli et al., 2005, Mameli and Valenzuela, 2006, Valenzuela et al., 2008). Therefore, alterations in the developmental actions of PregS at PCs may play a role in the pathophysiology of the cerebellar alterations that characterize fetal alcohol spectrum disorders.
Research Highlights
Pregnenolone sulfate increases glutamate release at developing Purkinje neurons
GABA release was also increased but to a lesser extent
The increase in glutamate release is selective for developing climbing fibers
The mechanism of action involves activation of a TRP channel
These effects may contribute to synapse stabilization in the developing cerebellum
Acknowledgement
We thank Dr. Don Partridge for critically reading the manuscript.
This work was supported by NIH Grants MH70386 and AA14973
List of Abbreviations
- ACSF
Artificial cerebrospinal fluid
- α7nACh
α7 nicotinic Acetylcholine
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- [Ca2+]i
Intracellular calcium concentration
- CF
Climbing Fiber
- DL-APV
DL-2-Amino-5-phosphonopentanoic acid
- eEPSC
Evoked excitatory postsynaptic current
- EGTA
ethylene glycol tetraacetic acid
- GABA
γ-Aminobutyric acid
- IPSC
Inhibitory postsynaptic current
- K-S test
Kolmogorov-Smirnov Test
- MLA
Methyllycaconitine
- mEPSC
Miniature excitatory postsynaptic current
- mIPSC
Miniature inhibitory postsynaptic current
- MLI
Molecular layer interneuron
- mPSCs
Miniature postsynaptic current
- NMDA
N-methyl-D-aspartic acid
- PC
Purkinje cell
- PF
Parallel Fiber
- PPR
Paired-pulse ratio
- PregS
Pregnenolone Sulfate
- TRP
Transient receptor potential
- TRPC
Transient receptor potential (canonical family)
- TRPM
Transient receptor potential (melastatin family)
- VGCCs
Voltage gated calcium channels
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akk G, Bracamontes J, Steinbach JH. Pregnenolone sulfate block of GABA(A) receptors: mechanism and involvement of a residue in the M2 region of the alpha subunit. J Physiol. 2001;532:673–684. doi: 10.1111/j.1469-7793.2001.0673e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Pucovsky V, Prestwich SA, Large WA. TRPC3 properties of a native constitutively active Ca2+-permeable cation channel in rabbit ear artery myocytes. J Physiol. 2006;571:361–369. doi: 10.1113/jphysiol.2005.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Bidoret C, Ayon A, Barbour B, Casado M. Presynaptic NR2A-containing NMDA receptors implement a high-pass filter synaptic plasticity rule. Proc Natl Acad Sci U S A. 2009;106:14126–14131. doi: 10.1073/pnas.0904284106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman LW, Konnerth A. Activity-dependent plasticity of developing climbing fiber-Purkinje cell synapses. Neuroscience. 2009;162:612–623. doi: 10.1016/j.neuroscience.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Bosman LW, Takechi H, Hartmann J, Eilers J, Konnerth A. Homosynaptic long-term synaptic potentiation of the "winner" climbing fiber synapse in developing Purkinje cells. J Neurosci. 2008;28:798–807. doi: 10.1523/JNEUROSCI.4074-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira JC, Wu Y, Mameli M, Purdy RH, Li PK, Akwa Y, Savage DD, Engen JR, Valenzuela CF. Fetal alcohol exposure alters neurosteroid levels in the developing rat brain. J Neurochem. 2004;90:1530–1539. doi: 10.1111/j.1471-4159.2004.02686.x. [DOI] [PubMed] [Google Scholar]
- Cesa R, Strata P. Axonal competition in the synaptic wiring of the cerebellar cortex during development and in the mature cerebellum. Neuroscience. 2009;162:624–632. doi: 10.1016/j.neuroscience.2009.02.061. [DOI] [PubMed] [Google Scholar]
- Chen L, Sokabe M. Presynaptic modulation of synaptic transmission by pregnenolone sulfate as studied by optical recordings. J Neurophysiol. 2005;94:4131–4144. doi: 10.1152/jn.00755.2004. [DOI] [PubMed] [Google Scholar]
- Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. J Physiol. 2008;vol. 586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Fu YM, Sun JL, Zhu YH, Sun FY, Zheng P. Neurosteroid enhances glutamate release in rat prelimbic cortex via activation of alpha1-adrenergic and sigma1 receptors. Cell Mol Life Sci. 2005;62:1003–1014. doi: 10.1007/s00018-005-5004-8. [DOI] [PubMed] [Google Scholar]
- Eilers J, Plant TD, Marandi N, Konnerth A. GABA-mediated Ca2+ signalling in developing rat cerebellar Purkinje neurones. J Physiol. 2001;536:429–437. doi: 10.1111/j.1469-7793.2001.0429c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs TT, Russek SJ, Farb DH. Sulfated steroids as endogenous neuromodulators. Pharmacology Biochemistry and Behavior. 2006;84:555. doi: 10.1016/j.pbb.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem. 2003;278:21493–21501. doi: 10.1074/jbc.M300945200. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hige T, Fujiyoshi Y, Takahashi T. Neurosteroid pregnenolone sulfate enhances glutamatergic synaptic transmission by facilitating presynaptic calcium currents at the calyx of Held of immature rats. Eur J Neurosci. 2006;24:1955–1966. doi: 10.1111/j.1460-9568.2006.05080.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Grimm C, Kraft R, Goldbaum O, Wrede A, Nolte C, Hanisch UK, Richter-Landsberg C, Bruck W, Kettenmann H, Harteneck C. TRPM3 is expressed in sphingosine-responsive myelinating oligodendrocytes. J Neurochem. 2010;114:654–665. doi: 10.1111/j.1471-4159.2010.06644.x. [DOI] [PubMed] [Google Scholar]
- Huang WC, Young JS, Glitsch MD. Changes in TRPC channel expression during postnatal development of cerebellar neurons. Cell Calcium. 2007a;42:1–10. doi: 10.1016/j.ceca.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007b;8:673–686. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- Ito-Ishida A, Miura E, Emi K, Matsuda K, Iijima T, Kondo T, Kohda K, Watanabe M, Yuzaki M. Cbln1 regulates rapid formation and maintenance of excitatory synapses in mature cerebellar Purkinje cells in vitro and in vivo. J Neurosci. 2008;28:5920–5930. doi: 10.1523/JNEUROSCI.1030-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Historical review of the significance of the cerebellum and the role of Purkinje cells in motor learning. Ann N Y Acad Sci. 2002;978:273–288. doi: 10.1111/j.1749-6632.2002.tb07574.x. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009;19:154–161. doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Kawa K. Acute synaptic modulation by nicotinic agonists in developing cerebellar Purkinje cells of the rat. J Physiol. 2002;538:87–102. doi: 10.1113/jphysiol.2001.012885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa K. Glycine facilitates transmitter release at developing synapses: a patch clamp study from Purkinje neurons of the newborn rat. Brain Res Dev Brain Res. 2003;144:57–71. doi: 10.1016/s0165-3806(03)00159-7. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Llano I, Armstrong CM. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R, Harteneck C. The mammalian melastatin-related transient receptor potential cation channels: an overview. Pflugers Arch. 2005;451:204–211. doi: 10.1007/s00424-005-1428-0. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Hashimoto K, Kano M, Takayama C, Sakimura K, Mishina M, Inoue Y, Watanabe M. Impaired parallel fiber-->Purkinje cell synapse stabilization during cerebellar development of mutant mice lacking the glutamate receptor delta2 subunit. J Neurosci. 1997;17:9613–9623. doi: 10.1523/JNEUROSCI.17-24-09613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Cho JH, Choi IS, Park HM, Lee MG, Choi BJ, Jang IS. Pregnenolone sulfate enhances spontaneous glutamate release by inducing presynaptic Ca(2+)-induced Ca(2+) release. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.07.057. [DOI] [PubMed] [Google Scholar]
- Mameli M, Botta P, Zamudio PA, Zucca S, Valenzuela CF. Ethanol decreases Purkinje neuron excitability by increasing GABA release in rat cerebellar slices. J Pharmacol Exp Ther. 2008;327:910–917. doi: 10.1124/jpet.108.144865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Carta M, Partridge LD, Valenzuela CF. Neurosteroid-induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. J Neurosci. 2005;25:2285–2294. doi: 10.1523/JNEUROSCI.3877-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Valenzuela CF. Alcohol increases efficacy of immature synapses in a neurosteroid-dependent manner. Eur J Neurosci. 2006;23:835–839. doi: 10.1111/j.1460-9568.2006.04597.x. [DOI] [PubMed] [Google Scholar]
- Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacol Ther. 2007;116:107–124. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DA, Carta M, Partridge LD, Covey DF, Valenzuela CF. Neurosteroids enhance spontaneous glutamate release in hippocampal neurons. Possible role of metabotropic sigma1-like receptors. J Biol Chem. 2002;277:28725–28732. doi: 10.1074/jbc.M202592200. [DOI] [PubMed] [Google Scholar]
- Morara S, van der Want JJ, de Weerd H, Provini L, Rosina A. Ultrastructural analysis of climbing fiber-Purkinje cell synaptogenesis in the rat cerebellum. Neuroscience. 2001;108:655–671. doi: 10.1016/s0306-4522(01)00433-x. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. A presynaptic action of the neurosteroid pregnenolone sulfate on GABAergic synaptic transmission. Mol Pharmacol. 2003;64:857–864. doi: 10.1124/mol.64.4.857. [DOI] [PubMed] [Google Scholar]
- Naylor J, Li J, Milligan CJ, Zeng F, Sukumar P, Hou B, Sedo A, Yuldasheva N, Majeed Y, Beri D, Jiang S, Seymour VA, McKeown L, Kumar B, Harteneck C, O'Regan D, Wheatcroft SB, Kearney MT, Jones C, Porter KE, Beech DJ. Pregnenolone sulphate- and cholesterol-regulated TRPM3 channels coupled to vascular smooth muscle secretion and contraction. Circ Res. 2010;106:1507–1515. doi: 10.1161/CIRCRESAHA.110.219329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols RA, Dengler AF, Nakagawa EM, Bashkin M, Paul BT, Wu J, Khan GM. A constitutive, transient receptor potential-like Ca2+ influx pathway in presynaptic nerve endings independent of voltage-gated Ca2+ channels and Na+/Ca2+ exchange. J Biol Chem. 2007;282:36102–36111. doi: 10.1074/jbc.M706002200. [DOI] [PubMed] [Google Scholar]
- Ohtsuki G, Hirano T. Bidirectional plasticity at developing climbing fiber-Purkinje neuron synapses. Eur J Neurosci. 2008;28:2393–2400. doi: 10.1111/j.1460-9568.2008.06539.x. [DOI] [PubMed] [Google Scholar]
- Perez-Velazquez JL, Valiante TA, Carlen PL. Modulation of gap junctional mechanisms during calcium-free induced field burst activity: a possible role for electrotonic coupling in epileptogenesis. J Neurosci. 1994;14:4308–4317. doi: 10.1523/JNEUROSCI.14-07-04308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piochon C, Irinopoulou T, Brusciano D, Bailly Y, Mariani J, Levenes C. NMDA receptor contribution to the climbing fiber response in the adult mouse Purkinje cell. J Neurosci. 2007;27:10797–10809. doi: 10.1523/JNEUROSCI.2422-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda S, Harada N, Tsutsui K. Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J Neurosci. 2007;27:7408–7417. doi: 10.1523/JNEUROSCI.0710-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scelfo B, Strata P. Correlation between multiple climbing fibre regression and parallel fibre response development in the postnatal mouse cerebellum. Eur J Neurosci. 2005;21:971–978. doi: 10.1111/j.1460-9568.2005.03933.x. [DOI] [PubMed] [Google Scholar]
- Schiess AR, Partridge LD. Pregnenolone sulfate acts through a G-protein-coupled sigma1-like receptor to enhance short term facilitation in adult hippocampal neurons. Eur J Pharmacol. 2005;518:22–29. doi: 10.1016/j.ejphar.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Liere P, Akwa Y, Rajkowski K, Griffiths W, Bodin K, Sjovall J, Baulieu EE. Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem Int. 2008;52:522–540. doi: 10.1016/j.neuint.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Sun Y, Singh BB. TRPC channels and their implication in neurological diseases. CNS Neurol Disord Drug Targets. 2010;9:94–104. doi: 10.2174/187152710790966650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais L, Hourez R, Bearzatto B, Gall D, Schiffmann SN, Cheron G. Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. Proc Natl Acad Sci U S A. 2007;104:9858–9863. doi: 10.1073/pnas.0607037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschemacher A, Kasparov S, Kravitz EA, Rahamimoff R. Presynaptic action of the neurosteroid pregnenolone sulfate on inhibitory transmitter release in cultured hippocampal neurons. Brain Res. 1997;772:226–232. doi: 10.1016/s0006-8993(97)00872-x. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Sakamoto H, Ukena K. Biosynthesis and action of neurosteroids in the cerebellar Purkinje neuron. J Steroid Biochem Mol Biol. 2003;85:311–321. doi: 10.1016/s0960-0760(03)00229-2. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Ukena K. Neurosteroids in the cerebellar Purkinje neuron and their actions (review) Int J Mol Med. 1999;4:49–56. [PubMed] [Google Scholar]
- Valenzuela CF, Partridge LD, Mameli M, Meyer DA. Modulation of glutamatergic transmission by sulfated steroids: role in fetal alcohol spectrum disorder. Brain Res Rev. 2008;57:506–519. doi: 10.1016/j.brainresrev.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Dufer M, Lis A, Flockerzi V, Philipp SE, Oberwinkler J. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- Watt AJ, Cuntz H, Mori M, Nusser Z, Sjostrom PJ, Hausser M. Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci. 2009;12:463–473. doi: 10.1038/nn.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]


