Abstract
The Drosophila leg imaginal disc consists of a peripheral region that contributes to adult body wall, and a central region that forms the leg proper. While the patterning signals and transcription factors that determine the identity of adult structures have been identified, the mechanisms that determine the shape of these structures remain largely unknown. The family of Rho GTPases, which consists of 7 members in flies, modulates cell adhesion, actomyosin contractility, protrusive membrane activity, and cell-matrix adhesion to generate mechanical forces that shape adult structures. The Rho GTPases are ubiquitously expressed and it remains unclear how they orchestrate morphogenetic events. The Rho guanine nucleotide exchange factors (RhoGEFs) and Rho GTPase activating proteins (RhoGAPs), which respectively activate and deactivate corresponding Rho GTPases, have been proposed to regulate the activity of Rho signaling cascades in specific spatiotemporal patterns to orchestrate morphogenetic events. Here we identify restricted expression of 12 of the 20 RhoGEFs and 10 of the 22 Rho RhoGAPs encoded in Drosophila during metamorphosis. Expression of a subset of each family of RhoGTPase regulators was restricted to motile cell populations including tendon, muscle, trachea, and peripodial stalk cells. A second subset was restricted either to all presumptive joints or only to presumptive tarsal joints. Depletion of individual RhoGEFs and RhoGAPs in the epithelium of the disc proper identified several joint-specific genes, which act downstream of segmental patterning signals to control epithelial morphogenesis. Our studies provide a framework with which to understand how Rho signaling cascades orchestrate complex morphogenetic events in multicellular organisms, and evidence that patterning signals regulate these cascades to control apical constriction and epithelial invagination at presumptive joints.
Keywords: Epithelial morphogenesis, apical constriction, epithelial invagination, dAP-2, RhoGAP68F, RhoGAP5A
INTRODUCTION
The Drosophila leg imaginal disc is composed of distinct cell populations that undergo spectacular, coordinated, rearrangements during larval development and metamorphosis to generate the complex morphology of adult legs and adjoining ventral thorax (Fristrom et al., 1978; von Kalm et al., 1995). During metamorphosis the pseudostratified epithelium of the leg imaginal disc everts and elongates by changes in epithelial cell shape (Fristrom and Fristrom, 1993) and by intercalation between cells (Taylor and Adler, 2008). Epithelial cells at presumptive joints constrict their apices and invaginate to promote joint morphogenesis (Mirth and Akam, 2002), while the distal tarsal joints of the leg are further sculpted by Jun-kinase (JNK)-reaper mediated apoptosis (Manjon et al., 2007).
The Drosophila leg harbors specialized cell populations that migrate extensively during metamorphosis. Some are specified in the disc proper, while others migrate into the leg imaginal disc from the trunk. The imaginal are connected to the larval epidermis by peripodial cells that form a hollow stalk. At the onset of metamorphosis, these peripodial stalk cells intercalate into the larval epidermis to facilitate disc eversion (Pastor-Pareja et al., 2004). Subsequently, the lateral margins of each disc, led by the peripodial stalk cells, crawl over the larval epidermis and fuse with the lateral margins of adjacent discs to “stitch together” the adult body wall in a process known as disc closure (Pastor-Pareja et al., 2004; Usui and Simpson, 2000). Underneath the disc proper the muscle founders fuse with surrounding myoblasts to form syncytial myotubes., These myotubes migrate and anchor at epidermal muscle attachment sites that are specified in the epithelium near presumptive joints to generate tendinous structures (Soler et al., 2004). A distinct population of tendon precursors invaginates and elongates distally from the distal tip of the leg to generate an internal hollow rod-like structure. To facilitate gas exchange, a primary tracheal tube invades into the leg imaginal disc from the trunk, elongates distally and forms an elaborate system of lateral branches. In the process, the tip cells of the tracheal branches migrate, elongate, and create tensile stresses that trigger tube elongation by stalk-cell intercalation (SCI) (Caussinus et al., 2008).
Many of the signals and transcription factors that pattern the leg, and the cellular machineries that generate mechanical forces that alter cell shape and motile behavior have been identified (reviewed in) (Kojima, 2004; Lecuit and Lenne, 2007; Montell, 2008). However, it remains unclear how patterning signals regulate these machineries to drive morphogenesis. It has been proposed that changes in adhesive properties and contractile behavior of epithelial cells can alter the topology of epithelial structures (Lecuit and Lenne, 2007). However, the precise mechanisms that can trigger these changes are only beginning to be unraveled. The rich repertoire of morphogenetic events that shape the developing Drosophila leg imaginal disc provides a highly tractable genetic model with which to identify morphogenetic regulators and characterize their mechanism of action in a complex multi-cellular environment.
Members of the Rho family of GTPases modulate processes that can affect cell shape and epithelial topology such as cell adhesion, actomyosin contractility, actin dynamics, and polarized vesicle transport (Fukata and Kaibuchi, 2001; Hall, 2005; Symons and Rusk, 2003; Van Aelst and Symons, 2002). The Drosophila genome codes for 7 RhoGTPases, including the canonical RhoGTPases Rho1, Rac1 and Cdc42, whose morphogenetic roles remain poorly characterized. Legs with reduced Rho1 activity form crooked and thickened proximal leg segments (Edwards and Kiehart, 1996; Ward et al., 2003) suggesting a role for Rho1 and its upstream regulators and downstream effectors in control of axis elongation by cell shape changes and cell intercalation. RhoGTPases are activated by the RhoGEFs, which promote the exchange of GDP for GTP, and are inactivated by the RhoGAPs, which promote the hydrolysis of GTP to GDP (Etienne-Manneville and Hall, 2002; Jaffe and Hall, 2005). The RhoGEFs and RhoGAPs modulate the activities of target RhoGTPases in a variety of pathways and regulate their interactions with downstream effectors. During development, RhoGEFs and RhoGAPs that are expressed in restricted patterns could regulate locally the activities of their target RhoGTPases and their downstream effectors (Bernards, 2003; Rossman et al., 2005). Localized changes in activity of these effectors could in turn locally alter the mechanical properties of epithelial cells and thereby the morphogenesis of adult structures. Recent studies have uncovered essential roles for several RhoGEFs and RhoGAPs in shaping the morphology of diverse epithelial structures such as the ventral groove (Dawes-Hoang et al., 2005; Fox and Peifer, 2007; Hacker and Perrimon, 1998), the segmental groove (Mulinari et al., 2008), the spiracles (Brodu and Casanova, 2006; Simoes et al., 2006), the Malpighian tubules (Denholm et al., 2005), and the salivary glands (Xu et al., 2008). Signals that pattern these structures control the expression pattern of a subset of the RhoGEFs and RhoGAPs in these epithelial derivatives suggesting that patterning signals control epithelial morphogenesis, at least in part, by regulating the activity of Rho signaling cascades. The leg imaginal discs provides an excellent model with which to interrogate the genetic control of tissue morphogenesis, yet the role of the RhoGEFs and RhoGAPs has not been systematically investigated in this system.
In this study, we examined the expression pattern of 35 of the 42 RhoGEFs and RhoGAPs encoded in Drosophila by whole-mount in situ hybridization during early pupal stages of leg development. In conjunction, we depleted the function of 33 of the 42 RhoGEFs and RhoGAPs by expressing inducible hairpin RNAs in the distal part of the leg and examined the requirements of these genes in leg morphogenesis. We find unique expression patterns of over half of these regulators (12/20 RhoGEFs, 10/22 RhoGAPs) in morphogenetically active cell populations including in presumptive tarsal joints. Our data suggest that tarsal joint morphogenesis is achieved, at least in part, through the patterned regulation of RhoGEFs and RhoGAPs expression in the epithelium of the leg imaginal disc. We further identify a crucial role for several joint-specific RhoGEFs and RhoGAPs in tarsal joint morphogenesis downstream of the signals that organize segmental pattern, which implicates novel pathways in the control of apical constriction and epithelial invagination.
RESULTS
Genes expressed in motile cell populations
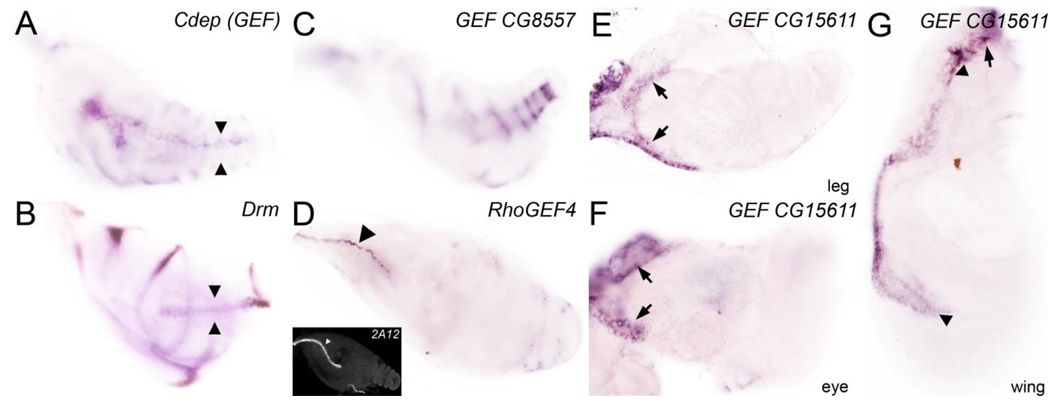
The developing Drosophila leg consists of several cell populations that migrate extensively during metamorphosis. We identified expression of 6 RhoGEF and 3 RhoGAP in these cell populations (Fig. 1 and Table 1). The RhoGEF Cdep was expressed within the leg shaft in a hollow tendinous structure, which invaginates and extends proximally from the distal tip during pupal stages (Fig. 1A). The drm gene is expressed similarly to Cdep in this tendinous structure (compare Fig. 1B with 1A; internal tendinous structure marked by arrowheads). RhoGEF CG8557 was expressed in a series of inner rings below the epithelium of the disc proper in a subpopulation of muscle founders that coalesces in a segmental pattern (Fig. 1C). dumbfounded-lacZ, which marks the entire muscle founder population, is expressed in a broader pattern (Soler et al., 2004). Several RhoGEFs and RhoGAPs were expressed in tracheal tubes that arborize within the leg during metamorphosis including RhoGEF4 (Fig. 1D), Ephexin (GEF), RhoGAP92B, and Graf (Table 1). The tracheal 2A12 antibody highlights a similar structure (inset in Fig. 1D). Finally, several RhoGEFs and RhoGAPs were restricted to peripodial stalk (PS) cells and the disc margin between the peripodial epithelium and the disc proper. Cells that localize to the disc margin, led by PS cells, crawls over the larval epidermis during metamorphosis to mediates disc closure. RhoGEF CG15611 was restricted to the disc stalk in legs, eyes, and wing imaginal discs, and to part of the disc margin (Fig. 1E, 1F, and 1G respectively). RhoGAP16F was restricted to the disc stalk of leg discs, suggesting a leg-specific role for this gene (Table 1). The subset of RhoGEFs and RhoGAPs that are expressed in motile cell populations could act along interdependent pathways to orchestrate the coordinated movements of cell clusters, sheets and tubes in order to generate specialized structures in adult legs.
Figure 1. A subset of RhoGEFs and RhoGAPs is expressed in motile cell populations.
(A) Cdep (GEF); expression is restricted to internal tendon precursor cells. (B) drm; expression is restricted to each true leg joint and to the internal tendon precursors (marked by arrowheads). (C) RhoGEF CG8557; expression is detected in a subset of adepithelial cells that form muscle below the surface epithelium. (D) RhoGEF4; expression is detected in a primary tracheal tube at the periphery of the leg. Tracheal antibody 2A12 highlights a tracheal tube in inset. (E–G) RhoGEF CG15611; Expression is detected in the disc stalk of the (E) leg disc, (F) antenna, (F) and in part of the ventral pleura in the wing (marked by arrowheads). These cell populations connect the imaginal disc to the larval epidermis and contribute to disc eversion, migration and fusion during metamorphosis to promote disc closure.
Table 1. Summary of the expression pattern and loss-of-function phenotypes of the RhoGEFs and RhoGAPs analyzed in this study.
For each gene, we describe the expression pattern in the leg and loss-of-function phenotypes induced by the expression of inducible hairpin RNAs with Dll-GAL4 or Dll-GAL4; UAS-Dicer. lacZ reporters that recapitulate endogenous gene expression are marked with asterisk. NP indicates no phenotype.
| Gene name | CG number | Leg Expression | RNAi phenotype |
|---|---|---|---|
| RhoGAP68F | CG6811 | Tarsal joints only | Shortened fused tarsal segments; partial joints |
| RhoGAP71E | CG32149 | Tarsal joints only * | Necrosis of distal leg |
| RhoGAP5A | CG3208 | Tarsal joints only | Fused tarsal segments; partial joints |
| RhoGEF2 | CG9635 | Tarsal joints only * | RNAi N/A |
| RhoGAP100F | CG1976 | Tarsal joints only | RNAi N/A |
| RhoGAP92B | CG4755 | Tarsal joints only; trachea | Bent tibia & femur |
| RhoGEF | CG30115 | All leg joints | Bent tibia & femur; tendon necrosis |
| vav GEF | CG7893 | All leg joints | NP |
| RhoGEF64C | CG32239 | All leg joints | Small leg stumps when raised at 18°C |
| RhoGAP15B | CG4937 | All leg joints | Bent tibia |
| RhoGAPp190 | CG32555 | Ubiquitous with elevated expression in all leg joints |
NP |
| trio GEF | CG18214 | All leg joints | Bent tibia & femur; slight thinning of tarsus |
| RhoGEF | CG7397 | All leg joints; trachea | NP |
| Ephexin GEF | CG3799 | All leg joints; trachea | Bent tibia & femur; tendon necrosis |
| Cdep GEF | CG31536 | Tendon precursor cells | NP |
| RhoGEF | CG8557 | Muscle precursor cells | Bent tibia & femur; tarsus bent & thinner |
| RhoGEF | CG15611 | Stalk and ventral pleura | NP |
| RhoGEF4 | CG8606 | Trachea | NP |
| Graf GAP | CG8948 | Ubiquitous with elevated expression in tarsal joints; trachea |
NP |
| RhoGAP16F | CG7122 | Stalk and trachea | Bent tibia & femur; tendon necrosis |
| RhoGAP19D | CG1412 | Ubiquitous | Bent tibia & femur |
| CdGAPr | CG10538 | Ubiquitous with elevated expression in tarsal joints* |
RNAi N/A |
| rtGEF | CG10043 | Ubiquitous | Larval lethal |
| RhoGEF3 | CG1225 | Ubiquitous | Larval lethal |
| RhoGEF | CG7323 | Ubiquitous | Bent tibia & femur |
| sif GEF | CG5406 | Ubiquitous | NP; maybe embryonic lethal |
| sos GEF | CG7793 | Ubiquitous with elevated expression in tarsal joints* |
No claw; fused tarsal segments; partial joints |
| RhoGEF | CG10188 | Low level ubiquitous | NP |
| RhoGEF | CG30456 | Low level ubiquitous | Bent tibia & femur |
| RhoGAP54D | CG6477 | Ubiquitous | Necrosis of tarsal joint |
| RhoGAP93B | CG3421 | Low level ubiquitous | NP |
| RhoGAP18B | CG7481 | Low level ubiquitous | NP; maybe embryonic lethal |
| RacGAP84C | CG2595 | Low level ubiquitous | NP |
| RhoGEF | CG33275 | Ubiquitous | Larval lethal; fused tarsal segments when raised at 18°C |
| cv-c | CG34389 | Ubiquitous | NP |
Joint-specific RhoGEFs and RhoGAPs
Joint morphogenesis is mediated by apical constriction and epithelial invagination, but the underlying mechanisms remain poorly understood. During joint morphogenesis the apical surface area of epithelial cells at presumptive joints decreases relative to those of adjacent cells that form the flanking leg segments (arrowheads in Fig. 5A-A’ point to apically constricted epithelial cells in presumptive joints). We have been particularly interested in identifying joint-specific regulators that could govern the progression of the process downstream of the signals that organize segmental pattern to better understand this morphogenetic process (Greenberg and Hatini, 2009).
Figure 5.
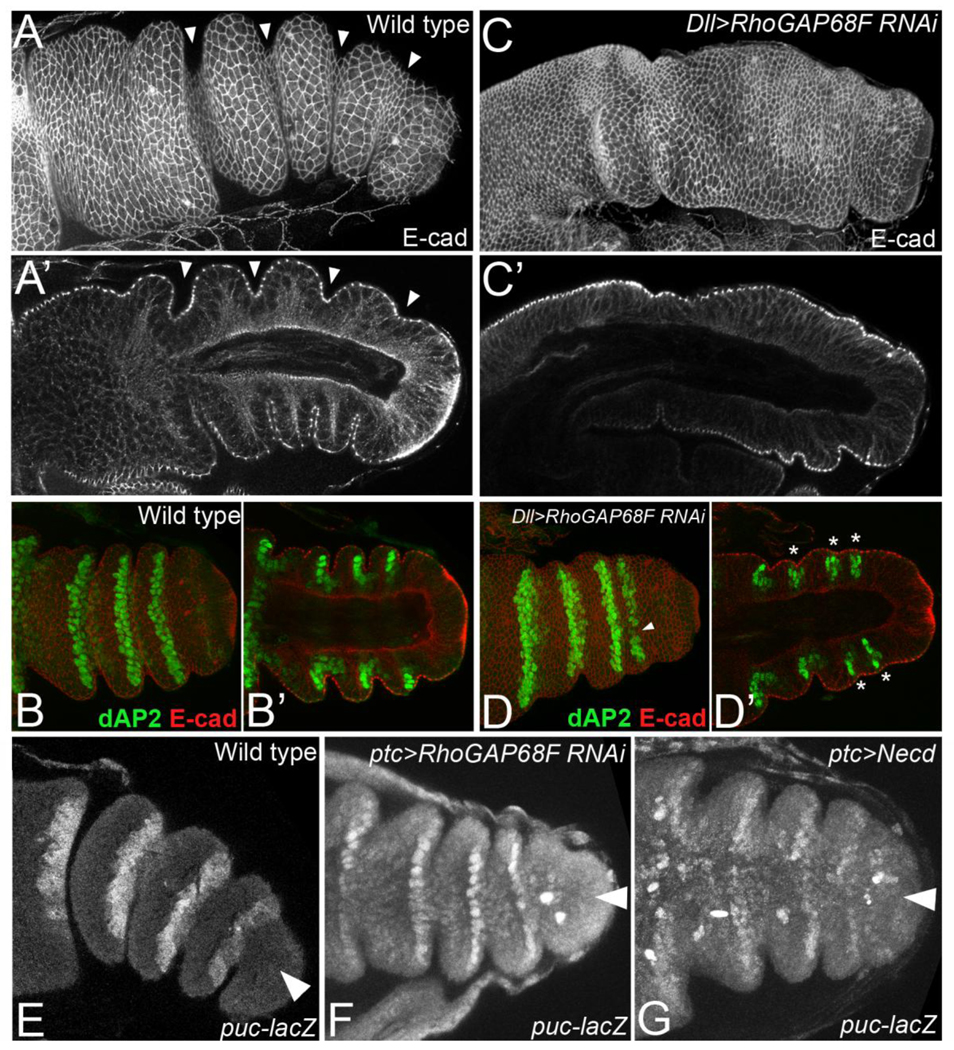
RhoGAP68F acts downstream of dAP-2 to promote apical constriction and epithelial invagination at presumptive joints. (A–B’) Wild type and (C–D’) Dll>RhoGAP68F RNAi leg imaginal disc stained for E-cad (white in A-A’ and C-C’, Red in B-B’ and D’-D) to mark cell outlines, and for dAP-2 (green in B-B’ and D’-D) to mark segment boundaries at ~4h after puparium formation (APF). (B and D) Grazing sections at the plane of the ZA (B’ and D’) and mid-saggital sections to reveal the apicobasal axis of the epithelium. (A-B’) The concentrically folded leg imaginal disc telescopes-out along the proximodistal (PD) axis and the epithelium of presumptive joints invaginates by apical constriction to initiate joint morphogenesis. dAP-2 accumulates at high levels in the distal part of the joint and at lower level in the proximal part. Note that proximal joints are more articulated compared to distal joints at this stage. (C–D’) Legs depleted for RhoGAP68F elongate along the PD axis but form either shallow or no invaginations at presumptive joints (asterisks in D’ indicate shallow invaginations). dAP-2 expression remains largely intact in these legs. In a small number of segments we detect small gaps or thinning (arrowheads in D) of the stripe of dAP-2 expression reflecting mild patterning defects. However, epithelial invaginations were either shallow or altogether missing despite the proper expression of dAP-2 in most segments indicating that RhoGAP68F acts downstream of dAP-2 to promote apical constriction and epithelial invagination.
(E–G) RhoGAP68F acts in parallel to JNK-reaper mediated apoptosis to promote tarsal joint morphogenesis. Arrowheads point to the Ptc domain. Expression of a puc-lacZ reporter in (E) wild type, (F) ptc>RhoGAP68FRNAi and (G) ptc>Necd (dominant negative N receptor). (E) The puc-lacZ reporter is expressed at high levels in tarsal joints 2–5 (F) Expression of RhoGAP68FRNAi with ptc-GAL4 led to a modest inhibition of epithelial invagination in the Ptc domain. However, puc-lacZ expression was not affected. . (G) In contrast, expression of Necd with ptc-GAL4 to inhibit N signaling in the Ptc domain led to the downregulation of puc-lacZ expression in the Ptc domain.
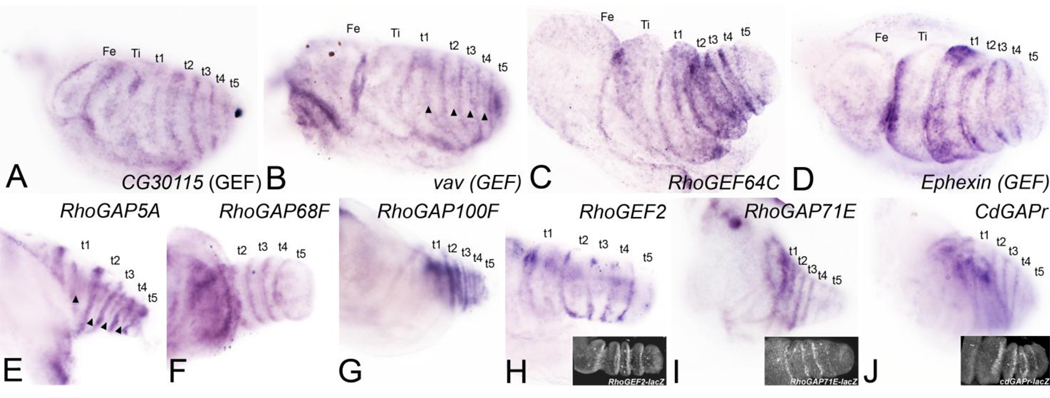
We found a total of 8 RhoGEFs and RhoGAPs restricted to all joints (Fig. 2A–D and Table 1) and 9 restricted to tarsal joints (Fig. 2E–J and Table 1). Available lacZ reporters for RhoGEF2, RhoGAP71E, and cdGAPr were expressed at presumptive joints in a similar pattern to the endogenous genes (insets in Fig. 2H, I, and J, respectively).
Figure 2. A subset of RhoGEFs and RhoGAPs is restricted to all presumptive leg joints or only to presumptive tarsal joints.
The leg imaginal disc gives rise to five true segments moveable by muscle: the coxa, trochanter, femur, tibia and tarsus. The tarsus is further subdivided into five non-musculated tarsal segments and a distal claw. (A–D) Genes restricted to all leg joints. (E-J) Genes restricted to tarsal joints. (A) CG30115 (RhoGEF). (B) vav (RhoGEF). (C) RhoGEF64C. (D) Ephexin (RhoGEF); expression spans the joint and several cell diameters proximal and distal to the joint. (E) RhoGAP5A. (F) RhoGAP68F (G). RhoGAP100F. (H) RhoGEF2; RhoGEF2-lacZ shown in inset. (I) RhoGAP71E; expression is stronger in proximal joints that are more articulated compared to distal joints that are less articulated, RhoGAP71E-lacZ shown in inset. (J) CdGAPr; CdGAPrlacZ shown in inset. A secondary stripe of expression is detected across each tarsal segment (marked by arrowheads) in B and E.
The segmental patterning system regulates the expression of the joint-specific RhoGAPs and RhoGEFs
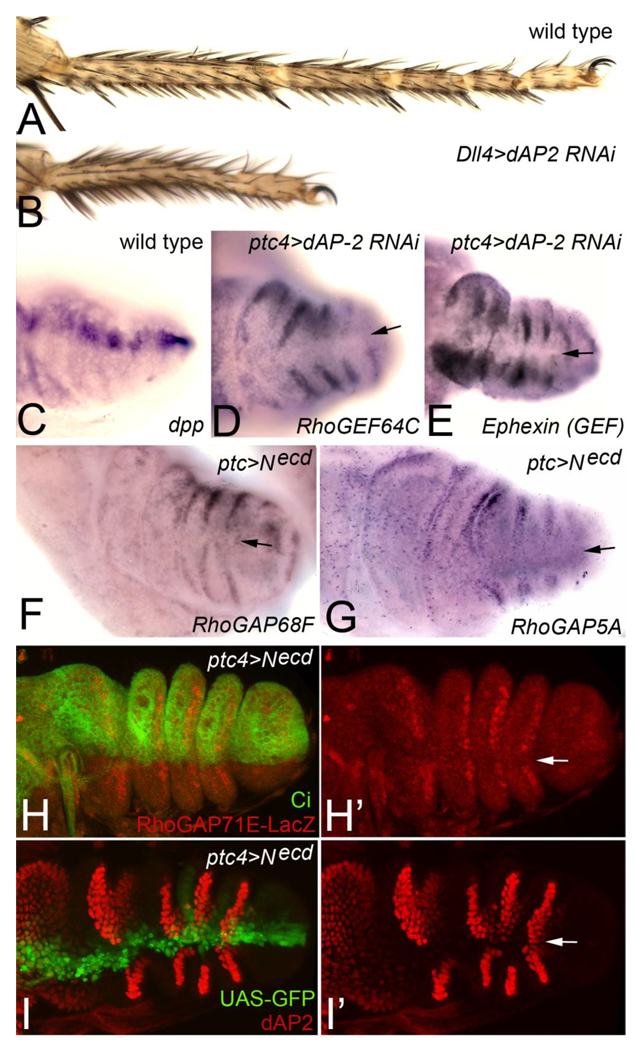
The expression of a large number of RhoGEFs and RhoGAPs at presumptive joints suggested that the segmental patterning system controls the expression of this subset of genes to promote joint morphogenesis. The Notch (N) receptor ligands Delta (Dl) and Serrate (Ser) are expressed at the distal end of each leg segment and signal to adjacent distal cells to induce the expression of several transcriptional regulators including dAP-2 (Ciechanska et al., 2007; Kerber et al., 2001) and bowl (Greenberg and Hatini, 2009). In turn, these transcriptional regulators promote leg segment growth and joint morphogenesis, though their downstream targets have not been identified. In addition, Dl and Ser induce low levels of target gene expression in adjacent proximal cells (Rauskolb and Irvine, 1999). dAP-2 and bowl also act cell-autonomously to repress Dl and Ser expression in the “N-activated region” to maintain a stable N signaling interface between Dl/Ser expressing cells and adjacent distal cells (Ciechanska et al., 2007; Greenberg and Hatini, 2009). The maintenance of this interface is crucial for leg segment growth and joint morphogenesis. The joint-specific RhoGEFs and RhoGAPs were expressed strongly distal to the Dl/Ser domain (Fig. 2) and some were expressed weakly proximal to this domain (Fig. 2B and 2E; secondary stripes indicated by arrowheads). To test if the N signaling interface is required to promote the expression of the joint-specific RhoGEFs and RhoGAPs genes, we expressed an inducible dAP-2 hairpin RNA transgene with ptc-GAL4 to de-repress Dl and Ser in the N activated region and thereby disrupt the N signaling interface in a narrow sector across each segment (marked by the expression of dpp in Fig. 3C). This led to gaps in expression of several representative joint-specific genes including RhoGEF64C and Ephexin (Fig. 3D and 3E, gaps in expression are marked by arrows). Broad expression of the dAP-2 RNAi transgene with Dll-Gal4 recapitulated the dAP-2 loss-of-function phenotype (Kerber et al., 2001), indicating that this RNAi transgene downregulates dAP-2 function specifically and effectively (Fig. 3B, compare to wild type in Fig. 3A). Likewise, the ectopic expression of dAP-2 with ptc-GAL4 to repress endogenous Dl and Ser expression led to gaps in RhoGEF68F and Ephexin expression in the Ptc domain (data not shown). Expression of a dominant negative Notch receptor (Necd) with ptc-GAL4 to inhibit N signaling in the Ptc domain led to similar gaps in expression of several representative joint specific genes including RhoGAP68F, RhoGAP5A and a lacZ reporter for RhoGAP71E. The expression of the RhoGAP71E-lacZ reporter was specifically downregulated in a narrow sector in the anterior compartment (marked by Ci expression) along the anteroposterior compartment boundary where Ptc is upregulated. Thus, the Notch pathway appears to promote the expression of this reporter cell autonomously. The expression of dominant negative N receptor led to similar gaps in expression of the N target dAP-2 in the Ptc domain. Taken together, these results indicate that N receptor signaling promotes the expression of the joint-specific RhoGAPs and RhoGEFs at presumptive joints either directly or indirectly.
Figure 3. Expression of joint-specific RhoGEFs and RhoGAPs requires the proper patterning of tarsal segments.
(A) Wild type adult tarsus. (B) Dll>dAP-2 RNAi; depletion of dAP-2 function with Dll-GAL4 resulted in shortened tarsus with fused joints that recapitulates a strong dAP-2 loss-of-function phenotype. (C) dpp is expressed along the AP compartment boundary where the ptc-GAL4 driver is active. (D–E) ptc>dAP-2 RNAi; depletion of dAP-2 in the Ptc domain resulted in repression of (D) RhoGEF64C and (E) Ephexin in a narrow sector marked by arrows. (F–I’) ptc>Necd; inhibition of N receptor signaling in the Ptc domain resulted in the repression of (F) RhoGAP68F, (G) RhoGAP5A, (H-H’) a RhoGAP71E-lacZ reporter and (I-I’) dAP-2 in a narrow sector marked by arrows. (H-H’) The expression of RhoGAP71E-lacZ was repressed in the anterior compartment (marked by Ci expression) along the AP compartment boundary. This narrow sector corresponds to the Ptc domain suggesting that N signaling promotes the expression of RhoGAP71E cell-autonomously. (I-I’) dAP-2 was repressed cell-autonomously in the Ptch domain validating the efficacy of the Necd transgene used in this assay (marked by UAS-GFP expression).
A subset of the joint-specific RhoGEFs and RhoGAPs is required to promote tarsal joint morphogenesis
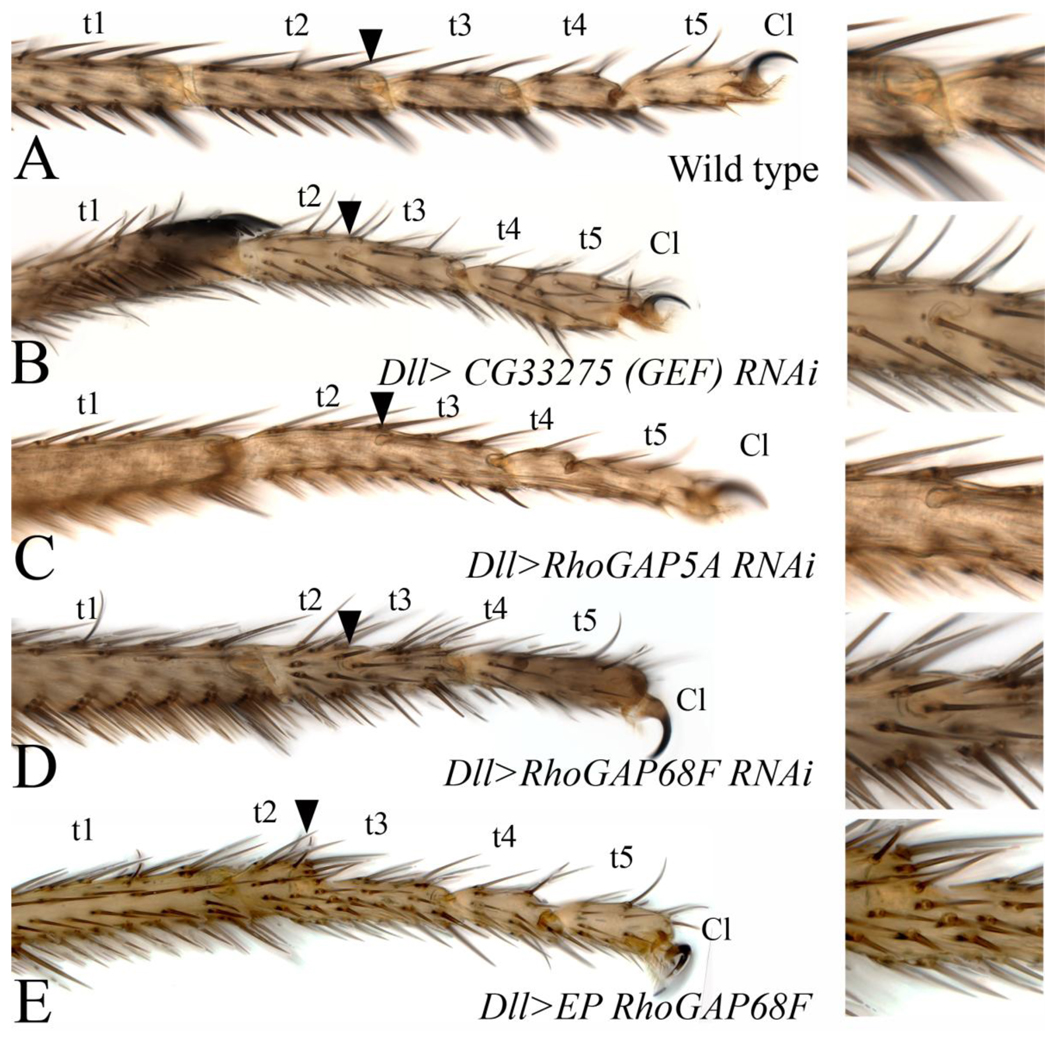
To identify novel regulators of epithelial morphogenesis and specifically those affecting apical constriction and epithelial invagination of presumptive joints, we depleted the function of individual RhoGEFs and RhoGAPs encoded in Drosophila by inducible hairpin RNA-mediated interference (RNAi) in the distal part of developing leg imaginal discs and assayed for phenotypes in adult legs (Bienz et al., 1988; Dietzl et al., 2007). In control experiments, we found that expression of hairpin RNAs to deplete a green fluorescent protein (GFP) caused no phenotypes (not shown). In contrast, depletion of several known regulators of leg development including bowl, son of sevenless (sos), Dachsous (Ds), and flamingo (fmi) by RNAi using leg-specific drivers recapitulated the respective mutant phenotype either fully or partially (Fig. 1 supplemental), thus validating the usefulness of this gene “knock down” strategy in the leg imaginal disc. Depletion of a subset of the RhoGEFs and RhoGAPs by RNAi, or by RNAi together with Dicer (Dicer RNAi) to enhance the production of short hairpin RNAs, resulted in several classes of reproducible phenotypes including distal leg truncations, necrosis of joints, bending and thickening of leg segments, and malformation and necrosis of internal structures (for data summary see Table 1 and Table 1 supplemental; for experimental protocol and scoring criteria of leg phenotypes see Materials and Methods). This range of phenotypes reveals important roles for these genes in leg development. Depletion of several tarsal joints-specific genes including RhoGEF CG33275, RhoGAP5A and RhoGAP68F (Table 1 and Figs. 2E and 2F, respectively) gave rise to a class of phenotypes characterized by missing or partially formed tarsal joints (Fig. 4B–D, compare to wild type in 4A) suggesting a specific role for this class of genes in apical constriction and epithelial invagination at presumptive joints. Consistent with this idea, depletion of these genes’ function had no adverse affects on the segmentation, size or differentiation of tarsal segments.
Figure 4. A subset of RhoGEFs and RhoGAPs is required for joint morphogenesis.
(A–E) Adult tarsi; (A) wild type; the tarsal region of adult legs is subdivided into 5 tarsal segments (t1–t5) and the distal claw (Cl). (B) Dll>CG33275 RNAi (RhoGEF), (C) Dll>RhoGAP5A RNAi and (D) Dll>RhoGAP68F RNAi. (B–D) Depletion of a subset of RhoGEFs and RhoGAPs by RNAi inhibits tarsal joint morphogenesis but does not adversely affect the growth and differentiation of leg segments. Arrowheads in B–D point to partially formed joints shown at higher magnification in insets. All the tarsal joints and tarsal segment (t1-t5) can be accounted for suggesting that the primary defect is in the progression of the process. Note that legs in B and D are slightly shorter and thicker than wild type reflecting a mild defect in axis elongation. (E) Dll>P{EP}RhoGAP68FP3152, Ectopic expression of RhoGAP68F also led to the formation of partially formed joints. Arrow in E points to such a joint.
To further characterize this phenotypic class, we examined the role of RhoGAP68F in epithelial morphogenesis in further detail. First, we examined epithelial morphology at metamorphosis in leg imaginal discs stained for E-cad to highlight cellular outlines and tissue contours. We found that the epithelium of the leg imaginal disc elongated along the proximodistal axis at metamorphosis and formed a tube-like structure with shallow or missing invaginations at presumptive joints (Fig. 5C-C’, compare to wild type in Fig. 5A-A’, and data not shown). To determine if RhoGAP68F affects joint morphogenesis after the establishment of tarsal segments, we stained legs depleted for RhoGAP68F for dAP-2 and E-cad to mark tarsal segment boundaries and highlight cell outlines, respectively. We observed largely normal expression of dAP-2 in most pupariating leg segments despite a block to apical constriction and epithelial invagination (Fig. 5D-D’, compare to wild type in 5B-B’, and data not shown; asterisks in D indicate presumptive joints that failed to invaginate). To determine if RhoGAP68F promotes joint morphogenesis indirectly by affecting JNK-reaper mediated apoptosis, we stained leg discs depleted for RhoGAP68F in the Ptc domain for a puckered (puc)-lacZ reporter, which mark JNK signaling activity and observed normal expression of this reporter in the Ptc domain despite a modest inhibition of epithelial invagination at presumptive joints (Fig 5F). In contrast, the expression of a dominant negative Notch receptor led to gaps in this reporters’ expression in the Ptc domain (compare Fig. 5G to wild type in 5E). These findings strongly suggest that RhoGAP68F acts downstream of segmental patterning signals and parallel to the JNK-reaper pathway to promote joint morphogenesis.
Several recessive lethal P-element insertions in the 5’ non-coding region of the RhoGAP68F gene have been identified including P{EP}RhoGAP68FEP3152, p{GSV7}GS20760/TM3 and P{GSV6}GS11699. We characterized the P{EP}RhoGAP68FEP3152 insertion in further detail. We find that 62% of P{EP}RhoGAP68FEP3152 homozygous animals (62 of 100 embryos analyzed) died during embryogenesis, while the remaining embryos that hatched died during the subsequent first larval instar stage. This lethal phase is consistent with a role for RhoGAP68F in facilitating epithelial morphogenesis during embryogenesis as has been previously reported (Sanny et al., 2006). The P{EP}RhoGAP68FEP3152 insertion contains UAS response elements that can be used to overexpress neighboring genes using the GAL4/UAS system. To examine the RhoGAP68F gain-of-function phenotype we crossed the P{EP}RhoGAP68FEP3152 line to Dll-GAL4 to broadly overexpress RhoGAP68F across the distal part of the leg. We found that the broad overexpression of RhoGAP68F also partially blocked the formation of tarsal joints (Fig. 4E), indicating that reduced or excess levels of RhoGAP68F impair joint morphogenesis.
Overall, we conclude that RhoGAP68F promotes apical constriction and epithelial invagination downstream of the signals that organize segmental pattern and specify the presumptive joints. We propose that RhoGAP68F in concert with other essential joint-specific genes including RhoGAP5A and RhoGEF CG33275 determine the mechanical properties of the epithelium at presumptive joints and thereby the topology of the epithelium in this region.
DISCUSSION
We identified restricted expression of a large number of RhoGEFs and RhoGAPs in regions and cell populations that are remodeled by changes in cell shape, cell-cell interaction, and cell motility, with several RhoGEFs and RhoGAPs typically expressed in any given region or cell type. These regulators are precisely positioned to control the modular mechanical forces (cell-cell adhesion, contractility, membrane protrusions, and cell-matrix interaction) generated by epithelial cells by regulating crucial downstream effectors to drive the morphogenesis of adult structure. N signaling promotes the expression of the joint-specific RhoGEFs and RhoGAPs suggesting that patterning signals drive joint morphogenesis, at least in part, by modulating the activity of Rho signaling cascades. A large number of RhoGEFs and RhoGAPs were expressed at presumptive joints and several of these genes were required for tarsal joint morphogenesis. We discuss the possible mechanism of action of these genes and their potential contribution to the process of apical constriction and epithelial invagination.
Control mechanisms of epithelial invagination at presumptive joints
Dl and Ser signal to adjacent distal cells to promote leg segment growth and joint morphogenesis. Dl and Ser induce the expression of several transcriptional regulators at presumptive joints including dAP-2 and the odd-skipped family genes drumstick (drm), oddskipped (odd), brother of odd and bowl with entrails limited (bowl), and sister of odd and bowl (sob). dAP-2 controls the formation of all the joints (Kerber et al., 2001; Monge et al., 2001), while the odd-skipped family genes appear to act redundantly to control the formation of non-tarsal joints, also termed true joints (Greenberg & Hatini, 2009). The mechanisms by which these signals and transcriptional regulator control epithelial morphogenesis have remained elusive. Localized JNK-reaper mediated apoptosis contributes to the articulation of presumptive joint by the localized elimination of epithelial cells in this region (Manjon et al., 2007). The activation of actomyosin contractility at presumptive joints has been proposed to promote apical constriction and epithelial invagination (Hao et al., 2003). The literature concerning the genes identified in our study such as RhoGAP5A and RhoGAP68F and their vertebrate homologs (Bruinsma et al., 2007; Sanny et al., 2006) suggests that the invagination of the epithelium at presumptive joints depends on additional mechanisms affecting junctional dynamics through the regulation of junction stability and trafficking itineraries of junction proteins as discussed below.
The control of junctional dynamics at presumptive joints
Emerging results suggest that the adherens junctions are the primary determinants of epithelial morphology (reviewed in) (Fernandez-Gonzalez and Zallen, 2008; Lecuit and Lenne, 2007; Warner and Longmore, 2009). The homophilic cell adhesion molecule-E-cadherin (E-cad) concentrates at the zonula adherens (ZA) below the apical cortex and links epithelial cells into a continuum of interconnected cells. Interacting E-cad molecules form a structure termed the adherens junction (AJ) that links interacting E-cad molecules to the actin and actomyosin cytoskeletal networks via several adaptor proteins to stabilize the ZA. Changes in cytoskeletal structure and dynamics can in principle enable epithelial cells to remodel cell-cell contacts, cell shape and epithelial topology. Like other transmembrane proteins, a fraction of E-cad molecules is constantly endocytosed and recycled to the plasma membrane. The modulation of this constitutive recycling pathway can also enables epithelial cells to remodel cell-cell contacts, cell shape and epithelial topology (Georgiou et al., 2008; Harris and Tepass, 2008; Leibfried et al., 2008).
During apical constriction the perimeter of the ZA appears to shrink, though the mechanisms involved remain poorly understood (Martin et al., 2009). The joint-specific RhoGEFs and RhoGAPs could affect the stability, trafficking and degradation of AJs in lysosomes to promote apical constriction. Consistent with this notion, the formation of the ventral furrow by apical constriction is associated with a large increase in endocytic internalization of AJs (Oda et al., 1998). Indirect evidence from the literature suggests that RhoGAP5A and RhoGAP68F (Fig. 2E & F, respectively) regulate adhesive cell-cell contacts by two distinct yet interdependent mechanisms. RhoGAP5A specifically inhibits Rac1, whose key function in epithelial cells is to stabilize cell-cell contacts by promoting the accumulation of actin filaments at AJs (Braga et al., 1999; Eaton et al., 1995; Takaishi et al., 1997). It is plausible that RhoGAP5A inhibits Rac1 at the ZA to decrease the accumulation of actin filaments at AJs in order to decrease the stability and increase the endocytic internalization of AJs at presumptive joints. Consistent with such a role, Chimaerins, the vertebrate homologs of RhoGAP5A, are recruited to the plasma membrane by the signal transducers phosphoinositide 3-kinase (PI3K) and phospholipase C-γ (Plc-γ), which are enriched in the ZA in epithelial cells (Yang and Kazanietz, 2007). RhoGAP5A promotes apical constriction and tube elongation of the salivary gland (Kolesnikov and Beckendorf, 2007), and the remodeling of cell-cell contacts in the fly eye (Bruinsma et al., 2007), suggesting a general role for RhoGAP5A in junctional remodeling in epithelial cells.
RhoGAP68F, which specifically deactivates Rho1 (Sanny et al., 2006), could regulate a subsequent step in the process. p50RhoGAP, the vertebrate homolog of RhoGAP68F, localizes to several endocytic compartments via its Sec14 lipid binding domain where it regulate endocytic trafficking (Sirokmany et al., 2006). The Sec14 domains of p50RhoGAP and RhoGAP68F are 43% identical and 64% similar suggesting related roles for RhoGAP68F in endocytic control. The Rho1 GTPase plays an important role in promoting the formation and movement of endosomes between compartments (Derivery et al., 2009; Gomez and Billadeau, 2009; Liu et al., 2009) and RhoGAP68F could inhibit this role of Rho1. By this mechanism RhoGAP68F could affect the turnover of E-cad or other junctional components and thereby cell shape and epithelial topology. RhoGAP68F promotes the formation of the ventral furrow during embryogenesis suggesting a general role for this regulator in apical constriction (Sanny et al., 2006).
The control of actomyosin contractility at presumptive joints
Although, we were unable to examine the role of RhoGEF2 by RNAi, it is plausible that this regulator acts either alone or with other regulators to promote actomyosin contractility. During ventral furrow formation, RhoGEF2 concentrates apically and activates Rho1 in this region to promote the constriction of the apical actomyosin meshwork that is anchored at the ZA. Active Rho1 activates the formin-family protein Diaphanous to promote polymerization of linear actin filaments that assemble into a contractile actomyosin meshwork. In addition, it promotes the phosphorylation of the regulatory light chain of Myosin II termed Spaghetti squash (Sqh) to upregulate actomyosin contractility. RhoGEF2 promotes epithelial invagination in other tissues during embryogenesis suggesting a general role for this gene in apical constriction (Dawes-Hoang et al., 2005; Fox and Peifer, 2007; Grosshans et al., 2005; Hacker and Perrimon, 1998; Kolsch et al., 2007; Mulinari et al., 2008). RhoGEF2 is upregulated at presumptive joints (Fig. 2H) and is thus positioned to upregulate actomyosin contractility in this region to initiate joint morphogenesis. RhoGEF64C colocalizes apically with RhoGEF2, and the two proteins have been proposed to activate Rho1 to promote apical constriction and epithelial invagination (Simoes et al., 2006). RhoGEF2 and RhoGAP64C could act along the same pathway to promote actomyosin contractility at presumptive joints. We note however, that the depletion of RhoGEF64C as well as several other genes including RhoGAP54D and RhoGAP71E (Table 1) adversely affected leg developmental suggesting earlier and/or more general roles for this subset of genes in epithelial morphogenesis. Additional work will be required to assign specific roles for these regulators in epithelial morphogenesis.
Overall, we propose that the activities of essential genes identified in our screen are coordinated to constrict the apical cell cortex, destabilize and decrease the surface expression of apical junctional proteins to promote apical constriction and epithelial invagination at presumptive joints. It has been shown that apical constriction occurs by the pulses of actomyosin contractility (Martin et al., 2009). While a subset of the essential genes identified in our screen such as RhoGEF2 might promote contractile pulses, others such as RhoGAP5A and RhoGAP68F might act in the refractory phases between pulses to remove junctional components and membrane from the apical cortex in order to consolidate the constricted state.
The potential role of RhoGTPase regulators that are expressed in motile cell populations
Although we were particularly interested in genes that regulate apical constriction and epithelial invagination, we identified several RhoGEFs and RhoGAPs that are expressed in motile cell populations. These genes may influence, in addition to cortical tension and cell adhesion, the protrusive membrane activity and cell-matrix adhesion required for the polarization, membrane extension, and forward movement of motile cell populations (Friedl and Gilmour, 2009; Montell, 2008). A distinct set of experimental tools will be required to investigate the roles of these genes’ function in morphogenesis of motile cell populations.
The results presented in this study indicate that the leg imaginal disc holds a great promise to reveal general mechanisms and regulatory logic of epithelial morphogenesis in multi-cellular organisms. Our findings expand the list of genes involved in control of apical constriction and epithelial invagination, and suggest the existences of novel pathways that contribute to the process. Additionally, our findings identify novel genes that could regulate epithelial sheet migration, disc closure, and tracheal tube elongation. The identification of these genes’ expression and function in leg development provides a resource with which to understand the role of Rho signaling cascades, their upstream regulators and downstream effectors in shaping the morphology of adult structures in multicellular organisms.
EXPERIMENTAL PROCEDURES
Fly Strains
The following fly lines were used in this study: RhoGAP71E-LacZ, RhoGEF2-LacZ, sos-lacZ, cdGAPr-lacZ, and rtGEF-lacZ (Bloomington and Szeged Stock Centers). dumbfounded-LacZ (gift from Sree Devi Menon) was used to mark muscle precursors. dAP-2 RNAi (VDRC 41130) and a dominant negative N receptor (Necd) were used to block leg segmentation. ptc-GAL4 was used to express the dAP-2 RNAi along the AP compartment boundary and Dll-GAL4 in the distal part of the leg. RNAi transgenes used to deplete the function of each RhoGEF and RhoGAP are listed in supplemental Figure 1. Dll-GAL4 and Dll-GAL4; UAS-Dicer lines were used to express each RNAi transgene in the distal region of the leg.
In situ hybridization and probe preparation
w− pupae were dissected 4–6 hours after puparium formation (APF) and processed for in situ hybridization (ISH) (Sullivan et al., 2000). cDNA vectors were either linearized at the 5' multiple cloning sites or the cDNA was PCR amplified with generic primer sets appropriate for each vector. Digoxigenin-labeled antisense RNA probes were transcribed with appropriate RNA polymerase (SP6, T7 or T3) according to the manufacturer's protocol (Roche). Yields of synthesized RNA were estimated by gel electrophoresis and the optimal probe concentration for ISH was determined empirically for each probe. Stacks of bright-field images were obtained using a Zeiss Axioscope 2+ and composite projections were generated using compositeZP. Figures were assembled and adjusted using Adobe Photoshop CS3.
cDNA clones were obtained from the Drosophila Gene Collection (DGC, http://www.fruitfly.org/DGC/index.html). Fully sequenced clones were used when available; otherwise, clones were obtained from the EST collection. The following cDNA clones were used for probe synthesis:
RhoGAPs
pBS-RhoGAP68F (LD02491), pBS-RhoGAP71E (LD04071), pOT2-RhoGAP5A (SD02309), pOT2-RhoGAP18B (LD25711), pOTB7-RacGAP84C (AT12815), pFlc-cv-c (RE02250), pOT2-Graf (LD28528), pOT2-RhoGAP15B (SD08167), pOT2-RhoGAPp190 (GH17919), pOT2-RhoGAP16F (SD04011), pOT2-CdGAPr (LD27836), pOT2-RhoGAP93B (SD01504), pOT2-RhoGAP100F (LP17760), pOTB7-RhoGAP92B (AT11177), pOTB7-GEF26 (AT08279), pFlc-RhoGAP19D (RH60035), pFlc-RhoGAP54D (RE04485).
RhoGEFs
pOT2-Cdep (SD09116), pOT2-CG30115 (GH16956), pOT2-CG30440 (LD43457), pOT2-vav (LD25754), pOT2-CG8557 (SD02996), pFlc-CG15611 (RE34668), pOT2-RhoGEF4 (LD45290), pBS-CG33275 (GM01778), pOT2-RhoGEF64C (GH26207), pOT2-RhoGEF2 (SD04476), pOT2-sos (GH01796), pOT2-CG10188 (GH26723), pOT2-CG15612 (SD09786), pOT2-trio (SD08659), pOT2-Ephexin (GH03693), pOT2-CG7397 (GH19526), pFlc-rtGEF (RE32772), pBS-RhoGEF3 (HL01913), pFlc-CG7323 (RH56938), pOT2-sif (GH10341). In situ hybridization with pOT2-drm (LD 26791) was used to mark the internal tendon precursors.
RNAi screen and criteria to identify RNAi phenotypes
Each RNAi transgene was expressed with Dll-GAL4 at 25°C either alone or in the presence of UAS-Dicer to reduce gene function further. In cases where the expression of the RNAi transgene resulted in embryonic or pupal lethality, the RNAi transgene was expressed at 18°C to deplete gene function to intermediate levels. For each cross, at least 20 prothoracic legs were mounted and analyzed using a compound light microscope by two independent observers. Each observer checked: 1) if all the leg segments where accounted for, if segments where missing or if the leg proximodistal axis was truncated; 2) if legs were shorter, thicker, or bent; 3) if joints were missing or if they were partially formed; 4) if legs formed necrotic structures or other anomalies in epithelial organization such as ectopic invagination or internal vesicular structures; and 5) if bristles formed and if the bristle pattern was disorganized. Phenotypes that were detected in at least 5/20 legs where scored as positive. In most cases, related phenotypes were detected in greater then 10 of the 20 legs scored.
Immunofluorescence and confocal imaging
Discs were fixed and stained according to standard protocols. The following antibodies were used: rabbit anti-β-galactosidase (Cappel), rat anti-Ci (DSHB)and mouse anti-tracheal system 2A12 (DSHB). Secondary antibody conjugated to Cy3 or Cy2 fluorophores (Jackson ImmunoResearch) were used at 1:150. Stained discs were scanned using a Zeiss LSM510 confocal microscope in multi-tracking mode.
Supplementary Material
Supplemental Table 1: Summary of the loss-of-function analysis of Drosophila RhoGEFs and RhoGAPs in the distal part of the leg imaginal disc
For each gene we provide the CG number, gene name, the VDRC insertion line used to deplete gene function, and the resulting phenotypes in the absence and presence of a UAS-Dicer transgenes at 25°C. Insertions that caused embryonic or larval lethality were reared at 18°C to reduce gene activity to intermediate levels in order to recover adult flies. At least 2 RNAi insertions were tested for each transgene when possible. See Material and Methods for the criteria used to score phenotypes.
Supplemental Figure 1: Depletion of several genes in developing leg imaginal discs recapitulated the loss-of-function phenotype. Each panels shows the morphology of the distal tarsus. (A) Wild type; (B) Dll>bowlRNAi, most tarsal segments were lost; (C) Dll>sos (RhoGEF); the claw and several distal tarsal segments were truncated and some remaining tarsal joints where partially formed; (D) Dll>DsRNAi; legs were shorter and intermediate tarsal joints were partially formed; (E) Dll>flmRNAi; tarsal joints were duplicated.
ACKNOWLEDGEMENTS
We thank the Drosophila Genomics Resource Center, the Bloomington Stock Center, the Vienna Drosophila Research Center (VDRC), the Szeged Drosophila Stock Center, M. Baylies, S. D. Menon, and the Developmental Studies Hybridoma Bank for cDNA, fly stocks, and antibodies, and K. Wharton and S. DelSignore for comments on the manuscript. This work was supported by a grant from the NIH to V.H. (R01GM06806).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta. 2003;1603:47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- Bienz M, Saari G, Tremml G, Müller J, Züst B, Lawrence PA. Differential regulation of ultrabithorax in two germ layers of drosophila. Cell. 1988;53:567–576. doi: 10.1016/0092-8674(88)90573-9. [DOI] [PubMed] [Google Scholar]
- Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10:9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodu V, Casanova J. The RhoGAP crossveinless-c links trachealess and EGFR signaling to cell shape remodeling in Drosophila tracheal invagination. Genes Dev. 2006;20:1817–1828. doi: 10.1101/gad.375706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma SP, Cagan RL, Baranski TJ. Chimaerin and Rac regulate cell number, adherens junctions, and ERK MAP kinase signaling in the Drosophila eye. Proc Natl Acad Sci U S A. 2007;104:7098–7103. doi: 10.1073/pnas.0701686104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caussinus E, Colombelli J, Affolter M. Tip-cell migration controls stalk-cell intercalation during Drosophila tracheal tube elongation. Curr Biol. 2008;18:1727–1734. doi: 10.1016/j.cub.2008.10.062. [DOI] [PubMed] [Google Scholar]
- Ciechanska E, Dansereau DA, Svendsen PC, Heslip TR, Brook WJ. dAP-2 and defective proventriculus regulate Serrate and Delta expression in the tarsus of Drosophila melanogaster. Genome. 2007;50:693–705. doi: 10.1139/g07-043. [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- Denholm B, Brown S, Ray RP, Ruiz-Gomez M, Skaer H, Hombria JC. crossveinless-c is a RhoGAP required for actin reorganisation during morphogenesis. Development. 2005;132:2389–2400. doi: 10.1242/dev.01829. [DOI] [PubMed] [Google Scholar]
- Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell. 2009;17:712–723. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Eaton S, Auvinen P, Luo L, Jan YN, Simons K. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J Cell Biol. 1995;131:151–164. doi: 10.1083/jcb.131.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KA, Kiehart DP. Drosophila nonmuscle myosin II has multiple essential roles in imaginal disc and egg chamber morphogenesis. Development. 1996;122:1499–1511. doi: 10.1242/dev.122.5.1499. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Zallen JA. Epithelial organization: may the force be with you. Curr Biol. 2008;18:R163–R165. doi: 10.1016/j.cub.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Fox DT, Peifer M. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development. 2007;134:567–578. doi: 10.1242/dev.02748. [DOI] [PubMed] [Google Scholar]
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- Fristrom D, Fristrom JW. The metamorphic development of the adult epidermis. In: Bate M, Martinez Arias A, editors. The Development of Drosophila Melanogaster. Cold Spring Harbor, New York: 1993. pp. 843–897. [Google Scholar]
- Fukata M, Kaibuchi K. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat Rev Mol Cell Biol. 2001;2:887–897. doi: 10.1038/35103068. [DOI] [PubMed] [Google Scholar]
- Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol. 2008;18:1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17:699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg L, Hatini V. Essential roles for lines in mediating leg and antennal proximodistal patterning and generating a stable Notch signaling interface at segment borders. Dev Biol. 2009;330:93–104. doi: 10.1016/j.ydbio.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans J, Wenzl C, Herz HM, Bartoszewski S, Schnorrer F, Vogt N, Schwarz H, Muller HA. RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development. 2005;132:1009–1020. doi: 10.1242/dev.01669. [DOI] [PubMed] [Google Scholar]
- Hacker U, Perrimon N. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 1998;12:274–284. doi: 10.1101/gad.12.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Hao I, Green RB, Dunaevsky O, Lengyel JA, Rauskolb C. The odd-skipped family of zinc finger genes promotes Drosophila leg segmentation. Dev Biol. 2003;263:282–295. doi: 10.1016/j.ydbio.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Kerber B, Monge I, Mueller M, Mitchell PJ, Cohen SM. The AP-2 transcription factor is required for joint formation and cell survival in Drosophila leg development. Development. 2001;128:1231–1238. doi: 10.1242/dev.128.8.1231. [DOI] [PubMed] [Google Scholar]
- Kojima T. The mechanism of Drosophila leg development along the proximodistal axis. Dev Growth Differ. 2004;46:115–129. doi: 10.1111/j.1440-169X.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Kolesnikov T, Beckendorf SK. 18 wheeler regulates apical constriction of salivary gland cells via the Rho-GTPase-signaling pathway. Dev Biol. 2007;307:53–61. doi: 10.1016/j.ydbio.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsch V, Seher T, Fernandez-Ballester GJ, Serrano L, Leptin M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science. 2007;315:384–386. doi: 10.1126/science.1134833. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol. 2008;18:1639–1648. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- Liu R, Abreu-Blanco MT, Barry KC, Linardopoulou EV, Osborn GE, Parkhurst SM. Wash functions downstream of Rho and links linear and branched actin nucleation factors. Development. 2009;136:2849–2860. doi: 10.1242/dev.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjon C, Sanchez-Herrero E, Suzanne M. Sharp boundaries of Dpp signalling trigger local cell death required for Drosophila leg morphogenesis. Nat Cell Biol. 2007;9:57–63. doi: 10.1038/ncb1518. [DOI] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirth C, Akam M. Joint development in the Drosophila leg: cell movements and cell populations. Dev Biol. 2002;246:391–406. doi: 10.1006/dbio.2002.0593. [DOI] [PubMed] [Google Scholar]
- Monge I, Krishnamurthy R, Sims D, Hirth F, Spengler M, Kammermeier L, Reichert H, Mitchell PJ. Drosophila transcription factor AP-2 in proboscis, leg and brain central complex development. Development. 2001;128:1239–1252. doi: 10.1242/dev.128.8.1239. [DOI] [PubMed] [Google Scholar]
- Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- Mulinari S, Barmchi MP, Hacker U. DRhoGEF2 and diaphanous regulate contractile force during segmental groove morphogenesis in the Drosophila embryo. Mol Biol Cell. 2008;19:1883–1892. doi: 10.1091/mbc.E07-12-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Tsukita S, Takeichi M. Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Dev Biol. 1998;203:435–450. doi: 10.1006/dbio.1998.9047. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Grawe F, Martin-Blanco E, Garcia-Bellido A. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev Cell. 2004;7:387–399. doi: 10.1016/j.devcel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Rauskolb C, Irvine KD. Notch-mediated segmentation and growth control of the Drosophila leg. Dev Biol. 1999;210:339–350. doi: 10.1006/dbio.1999.9273. [DOI] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Sanny J, Chui V, Langmann C, Pereira C, Zahedi B, Harden N. Drosophila RhoGAP68F is a putative GTPase activating protein for RhoA participating in gastrulation. Dev Genes Evol. 2006;216:543–550. doi: 10.1007/s00427-006-0067-6. [DOI] [PubMed] [Google Scholar]
- Simoes S, Denholm B, Azevedo D, Sotillos S, Martin P, Skaer H, Hombria JC, Jacinto A. Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis. Development. 2006;133:4257–4267. doi: 10.1242/dev.02588. [DOI] [PubMed] [Google Scholar]
- Sirokmany G, Szidonya L, Kaldi K, Gaborik Z, Ligeti E, Geiszt M. Sec14 homology domain targets p50RhoGAP to endosomes and provides a link between Rab and Rho GTPases. J Biol Chem. 2006;281:6096–6105. doi: 10.1074/jbc.M510619200. [DOI] [PubMed] [Google Scholar]
- Soler C, Daczewska M, Da Ponte JP, Dastugue B, Jagla K. Coordinated development of muscles and tendons of the Drosophila leg. Development. 2004;131:6041–6051. doi: 10.1242/dev.01527. [DOI] [PubMed] [Google Scholar]
- Sullivan W, Ashburner M, Hawley RS. Drosophila protocols. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Symons M, Rusk N. Control of vesicular trafficking by Rho GTPases. Curr Biol. 2003;13:R409–R418. doi: 10.1016/s0960-9822(03)00324-5. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Adler PN. Cell rearrangement and cell division during the tissue level morphogenesis of evaginating Drosophila imaginal discs. Dev Biol. 2008;313:739–751. doi: 10.1016/j.ydbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui K, Simpson P. Cellular basis of the dynamic behavior of the imaginal thoracic discs during Drosophila metamorphosis. Dev Biol. 2000;225:13–25. doi: 10.1006/dbio.2000.9766. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, Symons M. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev. 2002;16:1032–1054. doi: 10.1101/gad.978802. [DOI] [PubMed] [Google Scholar]
- von Kalm L, Fristrom D, Fristrom J. The making of a fly leg: a model for epithelial morphogenesis. Bioessays. 1995;17:693–702. doi: 10.1002/bies.950170806. [DOI] [PubMed] [Google Scholar]
- Ward RE, Evans J, Thummel CS. Genetic modifier screens in Drosophila demonstrate a role for Rho1 signaling in ecdysone-triggered imaginal disc morphogenesis. Genetics. 2003;165:1397–1415. doi: 10.1093/genetics/165.3.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner SJ, Longmore GD. Distinct functions for Rho1 in maintaining adherens junctions and apical tension in remodeling epithelia. J Cell Biol. 2009;185:1111–1125. doi: 10.1083/jcb.200901029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Keung B, Myat MM. Rho GTPase controls invagination and cohesive migration of the Drosophila salivary gland through Crumbs and Rho-kinase. Dev Biol. 2008;321:88–100. doi: 10.1016/j.ydbio.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Yang C, Kazanietz MG. Chimaerins: GAPs that bridge diacylglycerol signalling and the small G-protein Rac. Biochem J. 2007;403:1–12. doi: 10.1042/BJ20061750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Summary of the loss-of-function analysis of Drosophila RhoGEFs and RhoGAPs in the distal part of the leg imaginal disc
For each gene we provide the CG number, gene name, the VDRC insertion line used to deplete gene function, and the resulting phenotypes in the absence and presence of a UAS-Dicer transgenes at 25°C. Insertions that caused embryonic or larval lethality were reared at 18°C to reduce gene activity to intermediate levels in order to recover adult flies. At least 2 RNAi insertions were tested for each transgene when possible. See Material and Methods for the criteria used to score phenotypes.
Supplemental Figure 1: Depletion of several genes in developing leg imaginal discs recapitulated the loss-of-function phenotype. Each panels shows the morphology of the distal tarsus. (A) Wild type; (B) Dll>bowlRNAi, most tarsal segments were lost; (C) Dll>sos (RhoGEF); the claw and several distal tarsal segments were truncated and some remaining tarsal joints where partially formed; (D) Dll>DsRNAi; legs were shorter and intermediate tarsal joints were partially formed; (E) Dll>flmRNAi; tarsal joints were duplicated.