Abstract
Neurons in the dorsal root ganglia (DRG) are composed of a variety of sensory modalities, three of which are pain-sensing nociceptors, temperature-sensing thermoceptors, and itch-sensing pruriceptors. All these neurons are emerged from a common pool of embryonic DRG neurons that are marked by the expression of the neurotrophin receptor TrkA. Here we discuss how intrinsic transcription factors interface with target-derived signals to specify these functionally distinct sensory neurons. We will also discuss how this control mechanism provides a developmental perspective for the coding of somatic sensations.
Introduction
Somatic sensory neurons in dorsal root ganglia (DRG) transmit sensory information from the skin, bones, muscles, and visceral organs. These neurons are classified into several major subtypes, including i) proprioceptors that sense body positions, ii) low threshold mechanoreceptors that sense touch, pressure and vibration, iii) thermoceptors that respond to innocuous cold or warm temperatures, iv) nociceptors that respond to pain-inducing high threshold stimuli, and v) pruriceptors that respond to itch-inducing compounds. Each of these major sensory modalities represents a heterogeneous population of neurons. For example, different classes of nociceptors can be distinguished by differential responses to noxious mechanical, thermal or chemical stimuli [1].
Studies in the past century have suggested that the perception of specific somatic sensory modalities might be best explained by the population-coding (also called selectivity) hypothesis [2]. This hypothesis is composed of the following two features. First, each sensory modality is processed along a specific sensory circuit or labeled line, from the skin all the way to the brain. The existence of such labeled lines was suggested by a series of experiments carried out by Blix, Goldscheider, and Donaldson from 1882 to1885, and later by von Frey [3,4]. They observed that there are specific spots in the human skin whose activation evokes a specific sensation: cool, warm, touch, pain or itch. Modern molecular biology studies have led to the identification of sensory channels and receptors that are activated by specific thermal and/or chemical stimuli [1]. Most recently, cell ablation experiments have revealed a group of spinal cord neurons dedicated to itch sensation, providing a strong support for the existence of specific sensory labeled lines [5]. Second, a given sensory stimulus often activates multiple labeled lines; as a result, the coding of somatic sensation involves with a crosstalk between labeled lines [2]. For example, innocuous cold is able to activate both cool-sensing and pain-sensing sensory fibers, but pain can be masked by the activation of the cool labeled line, thereby leading to a pure cool sensation [2,4].
Key questions in the sensory neuron development field include the followings. How are specific labeled lines assembled? How could a given sensory receptor be associated with multiple sensory labeled lines? What are the mechanisms that lead to polymodal nature of many sensory neurons? Several recent reviews have extensively discussed the development of proprioceptors and sensori-motor circuits [6–8]. Here we will focus on the development of thermoreceptors, nociceptors, and pruriceptors.
Genesis of sensory neurons
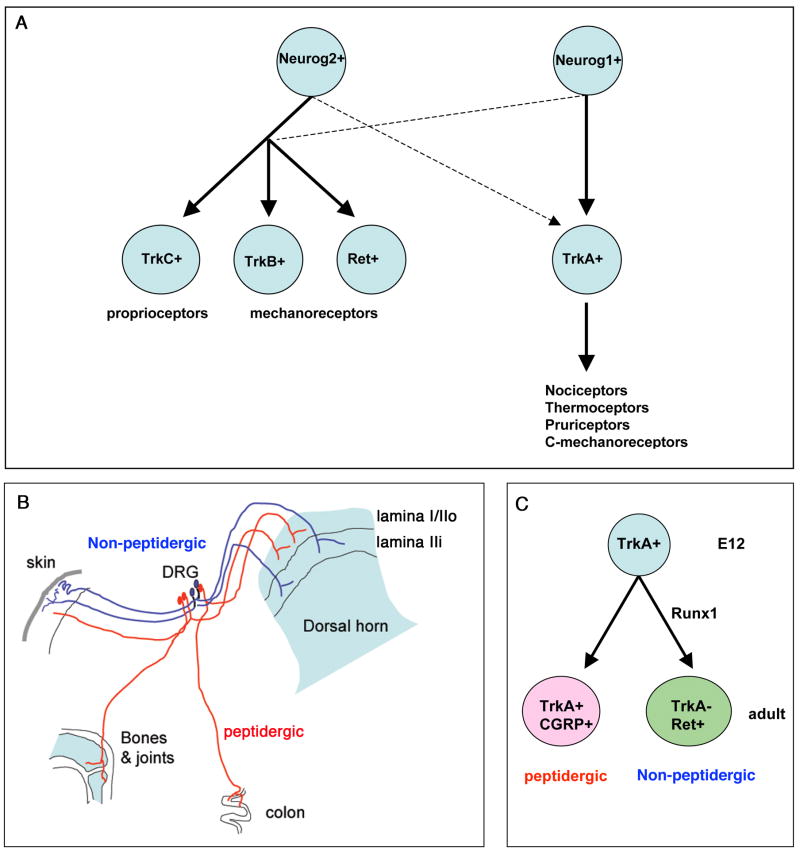
Sensory neurons in DRG are formed from the neural crest cells (NCCs) that migrate out of the dorsal neural tube [6]. The segregation of major classes of sensory neurons, such as proprioceptors/mechanoreceptors versus thermoceptors/nociceptors/pruriceptors can be traced to the very beginning of sensory neurogenesis (Figure 1A) [6]. The first wave of neurogenesis gives rise to large myelinated DRG neurons (referred to as Aβ-fiber neurons), including i) proprioceptors that are marked by the expression of TrkC (the receptor for the neurotrophin 3 or NT-3) [6], and ii) mechanoreceptors that marked by the expression of TrkB (the receptor for the brain-derived growth factor or BDNF) or Ret (the receptor for the glial-derived growth factor or GDNF family of neurotrophin factors) [6,9–11]. The second and third waves of neurogenesis give rise to non-myelinated or thinly myelinated DRG neurons (referred to as C-fiber and Aδ-fiber neurons, respectively), all of which are initially marked by the expression of TrkA (the receptor for the nerve growth factor or NGF) [6]; these late-born neurons will become nociceptors, thermoceptors, pruriceptors, and C-fiber low threshold mechanoreceptors or C-mechanoreceptors (Figure 1A).
Figure 1.
Genesis and segregation of major classes of sensory neurons. (A) Differential roles of Neurog1 and Neurog2 in controlling the genesis of major categories of sensory neurons. Solid and dashes arrows indicate the major and minor contributions, respectively. (B) Differential innervations of peptidergic and non-peptidergic neurons to peripheral and central targets. (C) Embryonic TrkA+ neurons give rise to adult TrkA+ peptidergic and Ret+ non-peptidergic neurons, and Runx1 is essential for the formation of Ret+ non-peptidergic neurons.
Genetic studies show that sensory neurogenesis is controlled by two related proneural genes, Neurog1 and Neurog2, as indicated by a complete loss of DRG neurons in mice lacking both Neurog1 and Neurog2 [12]. In Neurog2 single mutant mice, there is an initial loss of TrkC+, TrkB+ and Ret+ mechanoreceptors, although the loss of TrkC+ and TrkB+ neurons is later compensated by neurons formed from Neurog1-expressing precursors [12]. In Neurog1 mutant mice, about 27–35% of TrkC+ and TrkB+ neurons are lost, and TrkA+ neurons fail to form in the trigeminal ganglia and cervical DRG, although a small subset of TrkA+ neurons is still formed in thoracic and lumbar DRG [12]. These studies suggest that formation of proprioceptors and large mechanoreceptors largely depends on Neurog2, with a minor contribution from Neurog1+ precursors. Conversely, formation of TrkA+ neurons completely depends on Neurog1 in trigeminal ganglia and cervical DRG, but on both Neurog1 and Neurog2 in more caudal DRG (Figure 1A).
While the proneural activity of Neurog1/2 is essential for the genesis of sensory neurons, these early differentiation programs are switched off as newly born sensory neurons undergo terminal differentiation. This transition is controlled by two homeobox class transcription factors, Brn3a and Islet1, which are expressed in all sensory neurons and are required to suppress the expression of Neurog1/2 and their immediate downstream targets [13,14]. Brn3a and/or Islet1 are additionally required to suppress molecular programs that normally belong to the dorsal spinal cord, or cardiac and cranial mesodermal tissues [13,14], further indicating a critical role of Brn3a and Islet1 in establishing sensory neuron identity. In the remaining part of this review, we will discuss how embryonic TrkA+ neurons will progressively segregate into a heterogeneous group of sensory neurons involved in sensing pain, itch, temperature, and touch.
Segregation of TrkA+ peptidergic versus Ret+ non-peptidergic neurons
Sensory neurons involved in sensing pain, temperature, and itch are classically divided into two major subtypes: peptidergic and non-peptidergic (Figure 1B). In adult mice, most peptidergic neurons are marked by the expression of TrkA as well as by the expression of classic neuropetides such as calcitonin gene-related peptide (CGRP) and substance P (SP) [1,15]. Non-peptidergic neurons express Ret, a subset of which can be labeled by the isolectin B4 (IB4) [1,15]. Anatomically, non-peptidergic neurons preferentially innervate the skin, whereas peptidergic fibers project to most parts of the body. Centrally, peptidergic and non-peptidergic fibers terminate in distinct lamina in the dorsal horn of the spinal cord (Figure 1B), thereby forming two distinct sensory pathways [1,15].
Both TrkA+ peptidergic and Ret+ non-peptidergic neurons develop from embryonic TrkA+ neurons, and the segregation occurs during perinatal and postnatal development [16,17] (Figure 1C). Several studies show that a dynamic expression of Runx1 is crucial for the segregation of these two populations of sensory neurons [18–21]. Runx1 belongs to a family of runt domain-containing transcription factors. Within DRG, expression of Runx1 is confined exclusively to ~90% of TrkA-expressing neurons at early embryonic stage, and its expression is initiated soon after the onset of TrkA expression [18,20,22–24]. During perinatal and postnatal development, Runx1 is extinguished in future TrkA+ peptidergic neurons, but persists in a large subset of Ret+ neurons [18]. Analysis of mice that removed Runx1 expression in sensory precursors shows that Runx1 is necessary for the expression of Ret and to suppress TrkA and CGRP [18,19]. In these Runx1 mutant mice, prospective Ret+ non-peptidergic nociceptors are switched to become TrkA+;CGRP+ peptidergic nociceptors, and project to the most superficial (rather than inner) lamina in the dorsal horn, as peptidergic neurons normally do in wild type mice [18,19]. Furthermore, a genetic prevention of Runx1 down-regulation leads to a complete loss of TrkA expression and a marked reduction of CGRP, suggesting that Runx1 extinction is a prerequisite for the establishment of peptidergic sensory neurons [20,21].
How does Runx1 suppress TrkA expression in Ret+ non-peptidergic neurons? Luo et al. suggests a feed-forward control mechanism [25]. They made a conditional knockout of Ret in sensory precursors and found that a loss of Ret leads to delayed extinction of TrkA expression, although they were unable to examine TrkA expression after postnatal day 14 (P14) due to early lethality of these mice [25]. In other words, Runx1 may first activate Ret, and Ret-mediated signaling subsequently suppresses TrkA (Figure 2). However, this feed-forward control mechanism was disputed by a recent study [26]. Golden et al. made a Ret conditional knockout by using the Nav1.8-Cre mice to remove Ret in nociceptors at perinatal stage [26]. These mice are viable, and it was found that TrkA expression is still extinguished in prospective non-peptidergic nociceptors, suggesting that Ret signaling after perinatal stages is dispensable for TrkA extinction. How are these conflicting observations reconciled? One possibility is that Ret activity operating at earlier embryonic stages (prior to Ret removal by Nav1.8-Cre at perinatal stage) is required to establish a competent state for subsequent down-regulation of TrkA expression. As a result, TrkA extinction is delayed in early Ret knockout mice, but not affected in late knockouts.
Figure 2.
Dynamic expression and functions of Runx1 and neurotrophin receptors during segregation of peptidergic versus non-peptidergic neurons. Dashed arrows indicate possible contributions.
How is Runx1 expression is prevented or extinguished in peptidergic neurons? Earlier studies suggest that Bone Morphogenetic Protein (BMP)-mediated signaling might be critical for peptidergic differentiation [27]. However, neither Runx1 extinction nor peptidergic differentiation is affected in conditional knockout mice in which Smad4, a key component of the BMP signaling, was removed in nociceptors at perinatal stage (by crossing Smad4 conditional null mice with Nav1.8-Cre mice) [28]. This finding suggests the existence of BMP-independent pathways in controlling peptidergic differentiation, although it has not yet been ruled out that Smad4-independent BMP signaling or BMP signaling operating at early embryonic stages may control peptidergic differentiation [28]. Most recently, Gascon et al. made a key breakthrough in addressing this question, by revealing an essential role of Met, a receptor tyrosine kinase that binds to the hepatocyte growth factor (HGF), in synergy with TrkA, in controlling Runx1 extinction in a subset of peptidergic neurons (Figure 2) [29].
Gascon et al. found that TrkA+;CGRP+ peptidergic neurons could be generated from different lineages.(Figure 2). The first group expresses neither Met nor Ret, and emerges during early embryonic development (around E14.5-E16.5). Most likely, these early peptidergic neurons develop from ~10% of TrkA+ neurons that do not express Runx1 at E14.5 [18]. Since Runx1 expression is eliminated completely in Neurog1 mutant mice [20], those Runx1-negative TrkA+ neurons that are observed in thoracic/lumbar DRG in these mutant mice must be derived from Neurog2+ precursors, although a subset of these early peptidergic neurons could develop from Neurog1+ precursors that undergo rapid extinction of Runx1 expression (Figure 3). The second group of peptidergic neurons is marked by the expression of Met. In these neurons, Met, in synergy with TrkA, is required for Runx1 extinction (Figure 2). In Met conditional knockout mice (by crossing Met conditional null mice with Nestin-cre mice), prospective Met+ neurons retain Runx1 expression, which in turn leads to impaired peptidergic differentiation, as indicated by a loss of CGRP expression in this specific subpopulation [29]. Conversely, Runx1 is required to suppress Met expression in non-peptidergic neurons [29]. Thus, Met and Runx1 act as opposing genetic switches in establishing a non-peptidergic versus a peptidergic sensory neuron cell fate (Figure 2). The third group of peptidergic neurons co-expresses TrkA and Ret, but not Met, although it is not known if these neurons transiently express Met (Figure 2). Several outstanding questions remain. First, how is Met selectively activated and maintained in the second group of peptidergic neurons, and what signals cause Runx1 extinction in the third group of peptidergic neurons? Second, since peptidergic neurons innervate throughout the body, do these three groups of peptidergic neurons innervate distinct peripheral targets? Third, what are intrinsic transcription factors that are necessary for peptidergic neuron differentiation?
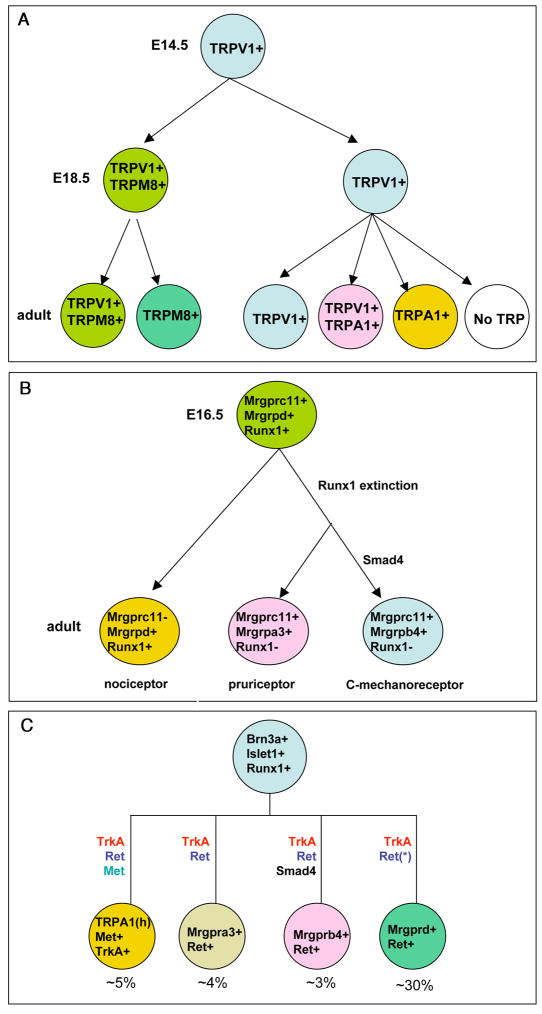
Figure 3.
Regulation of sensory channels and receptors. (A) Dynamic expression of TRP channels. (B) Progressive segregation of sensory modalities controlled by dynamic expression and dual activator/repressor activity of Runx1. Runx1 is a constitutive activator for Mrgprd, and Mrgprd expression is confined to Runx1-persistent neurons. Runx1 first acts as an activator for Mrgprc11, Mrgpra3, and Mrgprb4, but becomes a repressor at later stages; as a result, these genes can only be sustained in Runx1-transient neurons. The segregation of Mrgpra3+ and Mrgprb4+ neurons is further controlled by a selective involvement of Smad4-mediated BMP signaling in controlling Mrgprb4 expresssion (see also C). (C) Roles of target-derived signaling in regulating the expression of sensory channels and receptors. Note that expression of individual channels or receptors is dependent of specific combination of signaling pathways. “TRPA1(h)” means high level of TRPA1 expression. “Ret(*)” means that Ret is dispensable for Mrgprd expression, but essential for Mrgprd+ neurons to innervate the skin epidermis [25]. The percentages of DRG neurons expressing these channels/receptors are also shown.
Specification of sensory modalities
Individual sensory modalities are partly defined by the expression of sensory channels and receptors that allow them to respond to specific stimuli. In the past two decades, great achievement has been made in identifying such channels and receptors [1]. Examples include ATP-gated P2X class ion channels such as P2X3, transient receptor potential (TRP) ion channels (such as TRPV1, TRPA1 and TRPM8), and a dozen of Mrgpr class of G-protein coupled receptors or GPCRs (such as Mrgprd, Mrgpra3 and Mrgprb4) [1, 15]. These channels and receptors are associated with a cohort of functionally distinct sensory neurons (Figure 4) [1, 15]. TRPV1 responds to heat, proton, toxins, and the main chili pepper ingradient capsaicin [1]. TRPA1 responds to a range of chemical irritants and possibly to noxious cold as well, although the latter function remains controversial [1,30]. Mrgprd+ neurons are polymodal nociceptors that respond to noxious mechanical stimuli and heat [31,32]. Mrgpra3+ neurons function as pruriceptors and are required to sense itch evoked by chloroquine, a compound used to treat malaria but often causing itch side effects in human patients [33]. Mrgprb4+ neurons innervate exclusively the hairy skin and are speculated to function as low threshold C-fiber mechanoreceptors that might be involved in sensing pleasant touch [34].
There are other notable features of these sensory channels and receptors. First, while some of them are expressed exclusively in non-peptidergic neurons (such as Mrgprd and Mrgprb4) [31,34] or peptidergic neurons (such as high level of TRPA1 expression) [35], many others such as TRPM8 are expressed in both peptidergic and non-peptidergic neurons [36]. Second, expression of an individual channel or receptor can be specific for a single sensory modality (such as Mrgpra3 for chloroquine-induced itch) [33], but more often is associated with multiple sensory modalities (such as TRPM8 and TRPV1) [2]. For example, TRPM8+ cold-sensitive neurons are divided into two subtypes: Aδ-fiber neurons for cool sensation and C-fiber neurons likely for pain sensation [2]. Similarly, TRPV1+ neurons can be divided into pain-sensing and itch-sensing subpopulations [37–39]. Third, many sensory neurons are polymodal, by expressing multiple sensory channels and receptors. For example, a subset of TRPA1+ neurons and TRPM8+ neurons co-expresses TRPV1 [36,40–42]. Finally, sensory channels and receptors often show dynamic expression patterns during perinatal and postnatal development (Figures 3A and 3B) [40,42]. For example, TRPV1 is expressed broadly in embryonic TrkA+ neurons, and then progressively restricted to a small subset of DRG neurons (Figure 3A). Consistently, all TRPM8+ cold-sensitive neurons and TRPA1+ neurons initially respond to capsaicin, but a subset of them subsequently loses this responsiveness [40] (Figure 3A). Furthermore, TRPA1 expression in IB4− and IB4+ neurons emerges at P0 and P14, respectively [40].
In last several years, significant progresses have been made in understanding how such dynamic expression patterns of sensory receptors/channels are established during development. First of all, a series of transcription factors have been identified that play a pivotal role. Islet1, which is expressed in all DRG neurons, is required to set up the broad expression of TRPV1 in embryonic TrkA+ neurons [14]. Runx1, which is initially expressed in most TrkA+ neurons, coordinates the expression of a variety of sensory channels and receptors, including P2X3, TRPA1, TRPM8, the Mrgpr family of GPCRs, the sodium channel Nav1.9, and many others [18,21]. Brn3a is necessary for the expression of Runx1, thereby controlling many Runx1-dependent genes [43]. Additionally Brn3a controls Runx1-independent genes, such as the acid-sensing ion channel ASIC3 [43]. It should be noted that while Islet1 is required for both high (TRPV1high) and low levels of TRPV1 expression, Runx1 is required selectively to establish TRPV1high expression [14,18,21], providing an explanation why elevated TRPV1 expression is progressively confined to a small subset of DRG neurons. Conversely, while TRPM8 expression is entirely dependent on Runx1 and Brn3a, Islet1 is only required for the expression of a portion of TRPM8 [14,18,43]. It is, however, not known if Islet1 and Runx1 act in combination to control TRPM8 expression; nor is known if Islet1-dependent TRPM8+ neurons correspond to cool-sensing Aδ-fiber neurons or pain-sensing C-fiber neurons. These studies suggest that Runx1, Brn3a, and Islet1 coordinate the development of pain-sensing nociceptors (such as Mrgprd+, TrpA1+, TRPV1+ neurons), pruriceptors (Mrgpra3+ neurons), putative C-mechanoreceptors (Mrgprb4+ neurons), and others.
How could Runx1, Brn3a, and Islet1, which are broadly expressed in embryonic TrkA+ sensory neurons, be able to specify a range of functionally distinct sensory neurons that are marked by non-overlapping or partially overlapping expression of sensory channels and receptors? Runx1 can act both as a transcriptional activator and a repressor. It was further reported that Runx1 can switch from an activator to a repressor in regulating the same set of genes at different developmental stages [28]. This switch, combined with its persistent and transient expression in different populations of DRG neurons, provides one way for this single transcription factor to control the segregation of functionally distinct neurons, such as pain-sensing Mrgprd+ polymodal nociceptors versus Mrgrpa3+ pruriceptors, with the latter one also co-expressing Mrgprc11 [28] (Figure 3B). Initially, Mrgprd and Mrgprc11 are co-expressed in a large group of embryonic DRG neurons. However, at perinatal or postnatal stages, while Runx1 acts as a genetically constitutive activator for Mrgprd, it switches from a genetic activator to a repressor in regulating the expression of Mrgprc11, Mrgpra3 and Mrgprb4. As a result, Mrgprd expression can only be sustained in neurons with persistent Runx1 expression, whereas expression of Mrgprc11, Mrgrpa3, and Mrgprb4 can only be maintained in neurons with transient Runx1 expression (Figure 3B). The Runx1 repression activity is dependent on a c-terminal repression domain that binds to the Groucho co-repressor complex [28]. Removal of this domain results in an expansion of Mrgprc11, Mrgrpa3, and Mrgprb4 expression to Mrgprd+ neurons [28]. Conversely, a constitutive Runx1 expression in DRG neurons leads to a paradoxical loss of expression of Mrgprc11, Mrgrpa3, and Mrgprb4 [21]. Thus, dual activator/repressor activity plus dynamic expression of Runx1 provide an elegant way to control the segregation of functionally distinct sensory neurons (Figure 3B).
The next question is to understand how the dynamic activity and expression of these broadly distributed transcription factors are controlled. It should be noted that the expression of sensory channels and receptors is often established and refined at perinatal or postnatal stages, when axons have reached the peripheral targets [15]. Furthermore, genetic axon marking studies show that several Runx1-dependent genes, such as Mrgprd and Mrgprb4, are expressed in neurons innervating exclusively the skin epidermis and the hairy skin, respectively [31,34]. Based on this, target-specific signals could play an important role in regulating the expression and/or activity of intrinsic transcription factors. Indeed, several recent studies have revealed multiple signals in establishing the expression of Runx1-dependent genes (Figure 3C). First, TrkA signaling, which is required for axons to reach peripheral targets, is required for the expression of most Runx1-dependent genes [25]. Second, Ret-mediated signaling is required for the expression of a subset of Runx1-dependent gene, such as TRPA1, Mrgpra3 and Mrgprb4 [25]. Ret is dispensable for Mrgprd expression, but is essential for Mrgprd+ neurons to innervate the skin epidermis [28]. Third, Met signaling is required for the expression of a portion of TRPA1 and TRPV1high [29]. Finally, Smad4-dependent BMP signaling is required selectively for the expression of Mrgprb4 [28]. Notably, expression of different sensory channels and receptors is dependent of a distinct combination of signaling pathways (Figure 3C). However, future studies are needed to determine how exactly target-derived signals interface with broadly distributed transcription factors such as Runx1, Islet1, and Brn3a to control the expression of sensory channels and receptors in a specific subset of DRG neurons.
Assembly of specific sensory circuits or labeled lines
The coding of somatic sensation is most likely explained by the population-coding hypothesis, which highlights the existence of specific neural circuits or labeled lines that are specialized to transduce specific sensory modalities. The assembly of these labeled lines demands a coordination between i) the expression of a specific sensory receptor in a specific group of sensory neurons and ii) the ability of these neurons to make synaptic connections with a specific population of neurons in the dorsal spinal cord. To date, we know very little about how this coordination is achieved. One potential mechanism is that intrinsic transcription factors combined with specific target-derived signals may control the expression of both sensory channels/receptors and molecules involved in controlling axon targeting and synaptic connections. Support for this hypothesis includes the finding that Runx1 controls both the molecular identity of IB4+ non-peptidergic neurons and the innervation of these neurons to a specific dorsal horn lamina [18].
Implications on sensory coding
Specification of nociceptors, thermoceptors and pruriceptors is controlled by both i) intrinsic transcription factors that are expressed broadly in embryonic TrkA+ neurons, and ii) target-derived signals that likely act to modulate the expression and/or activity of intrinsic transcription factors. This control mechanism may provide a developmental perspective for the coding of somatic sensations. First, the finding that Runx1 controls both expression of channels/receptors in sensory neurons and central projections of these neurons may provide a foundation for the assembly of specific sensory labeled lines. Second, the fact that the same intrinsic transcription factors such as Runx1, Brn3a and Islet1 coordinate the development of a large cohort of functionally distinct sensory neurons may have the following consequences. Firstly, a given sensory receptor could be activated in neurons associated with distinct labeled lines. For examples, TRPV1 is expressed in both pain-sensing and itch-sensing neurons, and TRPM8 is expressed in Aδ-fiber cool-sensing neurons and C-fiber pain-sensing neurons. Secondly, multiple receptors could be expressed in the same neuron, thereby forming a basis for the polymodal nature of many DRG neurons. For example, TRPV1 and TRPM8 are co-expressed in a subset of cold-sensitive TRPM8+ neurons. Consequently, TRPM8 is associated with both Aδ cool-sensing fibers that express only TRPM8 and pain-sensing C-fibers that likely co-express TRPM8 and TRPV1. As a result, a salient feature of somatic sensory coding emerges: the responsiveness of a sensory fiber to a particular stimulus (such as innocuous cold) does not necessarily predict the sensory modality associated with this fiber (can be either cool or pain). Rather, the emergence of a specific sensation evoked by a stimulus (such as cool by innocuous cold) is involved with a crosstalk between distinct labeled lines in the central nervous system (such as a dominant suppression of pain by cool) [2]. Third, the involvement of target-derived signals in controlling the expression of sensory channels and receptors may explain why adult sensory neurons can undergo a wide range of phenotypic changes under pathological conditions, such as inflammation and nerve injuries, when sensory neurons are exposed to new environmental signals. Such plasticity may form a basis for the development of chronic inflammatory and neuropathic pain [1,15]. In this sense, two lines of research in the sensory neuron field: neural development and sensory coding can in fact meet and talk.
Acknowledgments
We thank Dr. Aziz Moqrich for his critical comments on this manuscript and for sharing a key manuscript prior to publication. We also thank our labmates for helpful discussions. Q.M. is supported by the NIH grants from NIDCR (1R01DE018025) and NINDS (5P01NS047572 and R01NS047710).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
- 1.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Q. Labeled lines meet and talk: population coding of somatic sensations. Journal of Clinical Investigation. 2010 doi: 10.1172/JCI43426. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norrsell U, Finger S, Lajonchere C. Cutaneous sensory spots and the “law of specific nerve energies”: history and development of ideas. Brain Res Bull. 1999;48:457–465. doi: 10.1016/s0361-9230(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 4.Green BG. Temperature perception and nociception. J Neurobiol. 2004;61:13–29. doi: 10.1002/neu.20081. [DOI] [PubMed] [Google Scholar]
- 5.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- 7.Dalla Torre di, Sanguinetto SA, Dasen JS, Arber S. Transcriptional mechanisms controlling motor neuron diversity and connectivity. Curr Opin Neurobiol. 2008;18:36–43. doi: 10.1016/j.conb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Zagami CJ, Zusso M, Stifani S. Runx transcription factors: lineage-specific regulators of neuronal precursor cell proliferation and post-mitotic neuron subtype development. J Cell Biochem. 2009;107:1063–1072. doi: 10.1002/jcb.22221. [DOI] [PubMed] [Google Scholar]
- 9.Luo L, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular Identification of Rapidly Adapting Mechanoreceptors and their Developmental Dependence on Ret Signaling. Neuron. 2009;64:841–856. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourane S, Garces A, Venteo S, Pattyn A, Hubert T, Fichard A, Puech S, Boukhaddaoui H, Baudet C, Takahashi S, et al. Low-threshold mechanoreceptor subtypes selectively express MafA and are specified by Ret signalling. Neuron. 2009;64:857–870. doi: 10.1016/j.neuron.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Honma Y, Kawano M, Kohsaka S, Ogawa M. Axonal projections of mechanoreceptive dorsal root ganglion neurons depend on Ret. Development. 2010;137:2319–2328. doi: 10.1242/dev.046995. [DOI] [PubMed] [Google Scholar]
- 12.Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes & Development. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanier J, Dykes IM, Nissen S, Eng SR, Turner EE. Brn3a regulates the transition from neurogenesis to terminal differentiation and represses non-neural gene expression in the trigeminal ganglion. Dev Dyn. 2009;238:3065–3079. doi: 10.1002/dvdy.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Sun Y, Dykes IM, Liang X, Eng SR, Evans SM, Turner EE. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nat Neurosci. 2008;11:1283–1293. doi: 10.1038/nn.2209. This study shows that Islet1, a transcription factor expressed in all sensory neurons, controls the transition from neurogenesis to terminal differentiation. Islet1 also controls sensory subtype specification by regulating the expression of sensory receptors and ion channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolf CJ, Ma Q. Nociceptors-noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Bennett DL, Averill S, Clary DO, Priestley JV, McMahon SB. Postnatal changes in the expression of the trkA high-affinity NGF receptor in primary sensory neurons. Eur J Neurosci. 1996;8:2204–2208. doi: 10.1111/j.1460-9568.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 17.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 18•.Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–377. doi: 10.1016/j.neuron.2005.10.036. This study shows that Runx1 selects a Ret+ non-peptidergic over a TrkA+ peptidergic cell fate, and also controls the expression of a large cohort of sensory channels and receptors. [DOI] [PubMed] [Google Scholar]
- 19•.Yoshikawa M, Senzaki K, Yokomizo T, Takahashi S, Ozaki S, Shiga T. Runx1selectively regulates cell fate specification and axonal projections of dorsal root ganglion neurons. Dev Biol. 2007;303:663–674. doi: 10.1016/j.ydbio.2006.12.007. This study shows that Runx1 selects a Ret+ non-peptidergic over a TrkA+ peptidergic cell fate. [DOI] [PubMed] [Google Scholar]
- 20•.Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron. 2006;49:379–393. doi: 10.1016/j.neuron.2006.01.008. This study shows that Runx1 extinction is essential for peptidergic neuron development. [DOI] [PubMed] [Google Scholar]
- 21.Abdel Samad O, Liu Y, Yang FC, Kramer I, Arber S, Ma Q. Characterization of two Runx1-dependent nociceptor differentiation programs necessary for inflammatory versus neuropathic pain. Mol Pain. 2010;6:45. doi: 10.1186/1744-8069-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levanon D, Bettoun D, Harris-Cerruti C, Woolf E, Negreanu V, Eilam R, Bernstein Y, Goldenberg D, Xiao C, Fliegauf M, et al. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J. 2002;21:3454–3463. doi: 10.1093/emboj/cdf370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theriault FM, Nuthall HN, Dong Z, Lo R, Barnabe-Heider F, Miller FD, Stifani S. Role for Runx1 in the proliferation and neuronal differentiation of selected progenitor cells in the mammalian nervous system. J Neurosci. 2005;25:2050–2061. doi: 10.1523/JNEUROSCI.5108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marmigere F, Montelius A, Wegner M, Groner Y, Reichardt LF, Ernfors P. The Runx1/AML1 transcription factor selectively regulates development and survival of TrkA nociceptive sensory neurons. Nat Neurosci. 2006;9:180–187. doi: 10.1038/nn1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A Hierarchical NGF Signaling Cascade Controls Ret-Dependent and Ret-Independent Events during Development of Nonpeptidergic DRG Neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. This study shows that TrkA and Ret signaling control a subset of Runx1-dependent sensory channels and receptors. [DOI] [PubMed] [Google Scholar]
- 26.Golden JP, Hoshi M, Nassar MA, Enomoto H, Wood JN, Milbrandt J, Gereau RWt, Johnson EMJ, Jain S. RET signaling is required for survival and normal function of nonpeptidergic nociceptors. J Neurosci. 2010;30:3983–3994. doi: 10.1523/JNEUROSCI.5930-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ai X, Cappuzzello J, Hall AK. Activin and bone morphogenetic proteins induce calcitonin gene-related peptide in embryonic sensory neurons in vitro. Mol Cell Neurosci. 1999;14:506–518. doi: 10.1006/mcne.1999.0798. [DOI] [PubMed] [Google Scholar]
- 28•.Liu Y, Yang FC, Okuda T, Dong X, Zylka MJ, Chen CL, Anderson DJ, Kuner R, Ma Q. Mechanisms of compartmentalized expression of Mrg class G-protein-coupled sensory receptors. J Neurosci. 2008;28:125–132. doi: 10.1523/JNEUROSCI.4472-07.2008. This study shows that dynamic expression plus dual activator/repressor activity of Runx1 are used to establish non-overlapping expression of sensory receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Gascon E, Gailard S, Malpert P, Liu Y, Radat-DEspoix L, Samokhvalov IM, Delmas P, Helmbacher F, Maina F, Moqrich A. HGF-Met signaling is required for Runx1 extinction and peptidergic differentiation in primary nociceptive neurons. Journal of Neuroscience. 2010 doi: 10.1523/JNEUROSCI.3135-10.2010. (In press). This study reveals three major categories of peptidergic neurons, and shows that Met signaling is essential for Runx1 extinction and for the formation of one group of peptidergic neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwan KY, Corey DP. Burning cold: involvement of TRPA1 in noxious cold sensation. J Gen Physiol. 2009;133:251–256. doi: 10.1085/jgp.200810146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005 Jan 6;45(1):17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Rau KK, McIlwrath SL, Wang H, Lawson JJ, Jankowski MP, Zylka MJ, Anderson DJ, Koerber HR. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. Journal of Neuroscience. 2009;29:8612–8619. doi: 10.1523/JNEUROSCI.1057-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q, Vrontou S, Rice FL, Zylka MJ, Dong X, Anderson DJ. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci. 2007;10:946–948. doi: 10.1038/nn1937. [DOI] [PubMed] [Google Scholar]
- 35.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 36.Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. Journal of Neuroscience. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shim WS, Oh U. Histamine-induced itch and its relationship with pain. Mol Pain. 2008;4:29. doi: 10.1186/1744-8069-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. This study reveals a progressive segregation of functionally distinct sensory neurons, as indicated by dynamic expression of TRP channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8- expressing sensory neurons and their projections. Journal of Neuroscience. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takashima Y, Ma L, McKemy DD. The development of peripheral cold neural circuits based on TRPM8 expression. Neuroscience. 2010;169:828–842. doi: 10.1016/j.neuroscience.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Dykes IM, Lanier J, Eng SR, Turner EE. Brn3a regulates neuronal subtype specification in the trigeminal ganglion by promoting Runx expression during sensory differentiation. Neural Dev. 2010;5:3. doi: 10.1186/1749-8104-5-3. This study shows that Brn3a, a transcription factor expressed in all sensory neurons, controls sensory subtype specification by regulating Runx1 and Ruxn3 expression. [DOI] [PMC free article] [PubMed] [Google Scholar]