Abstract
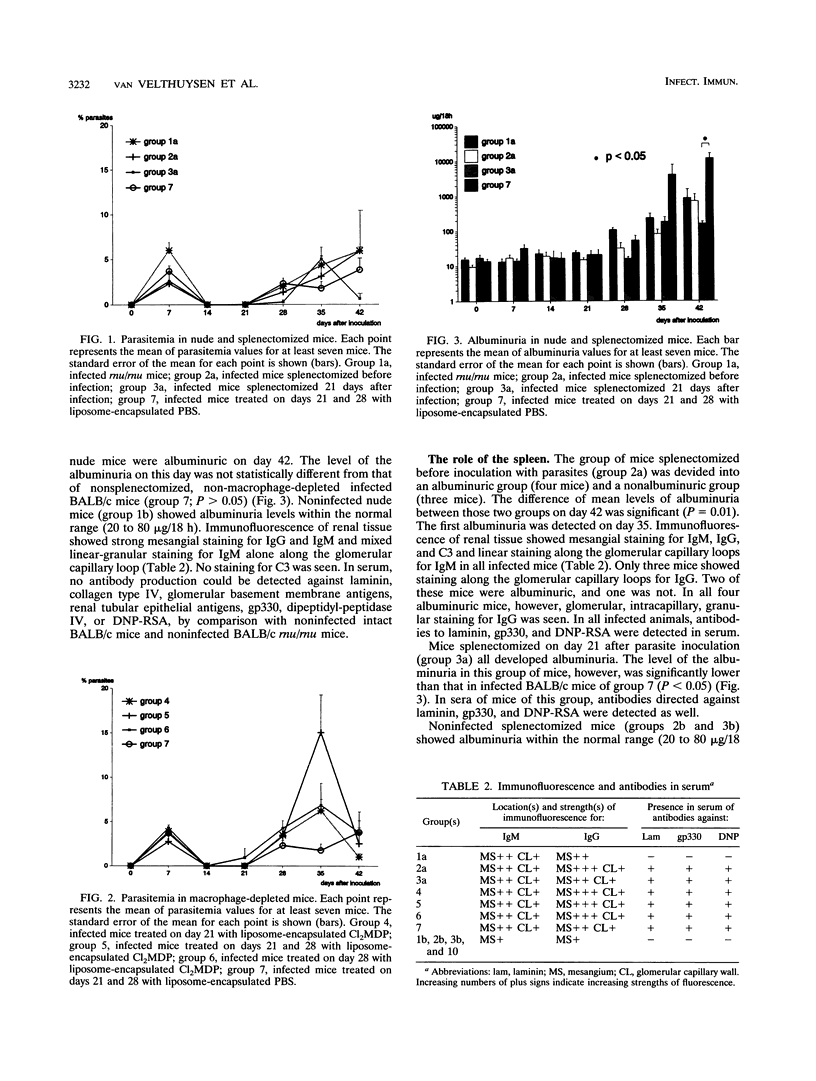
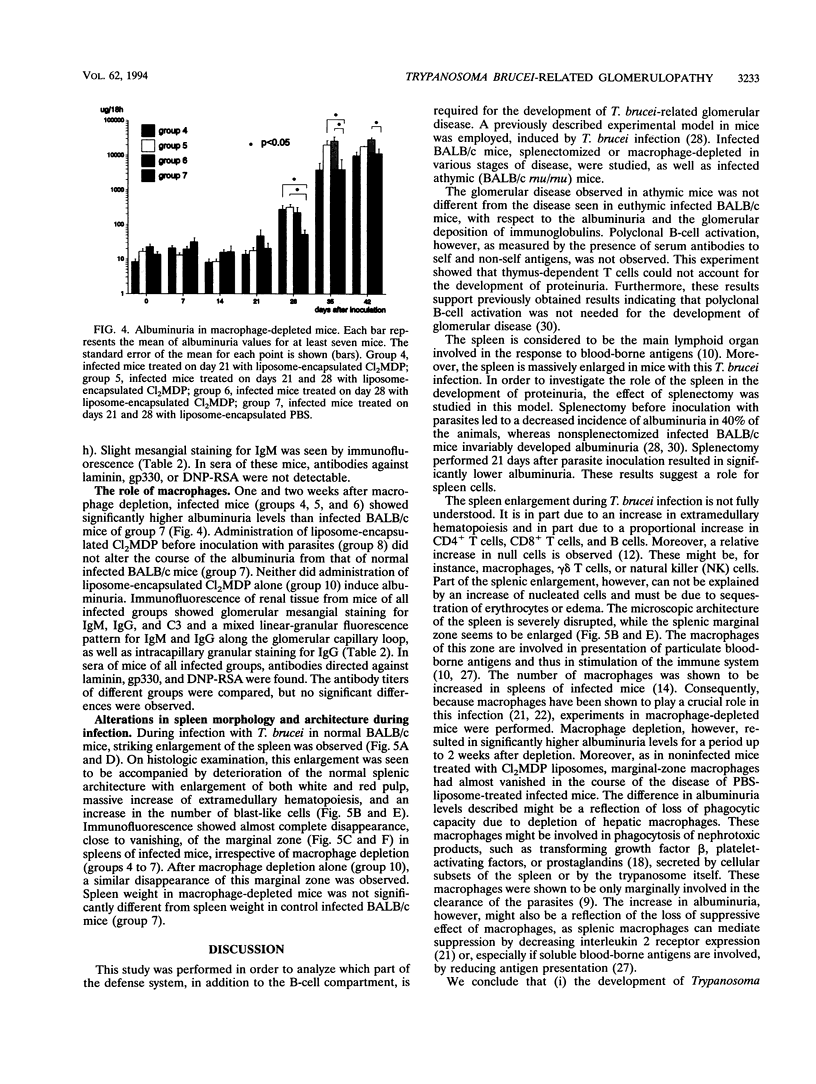
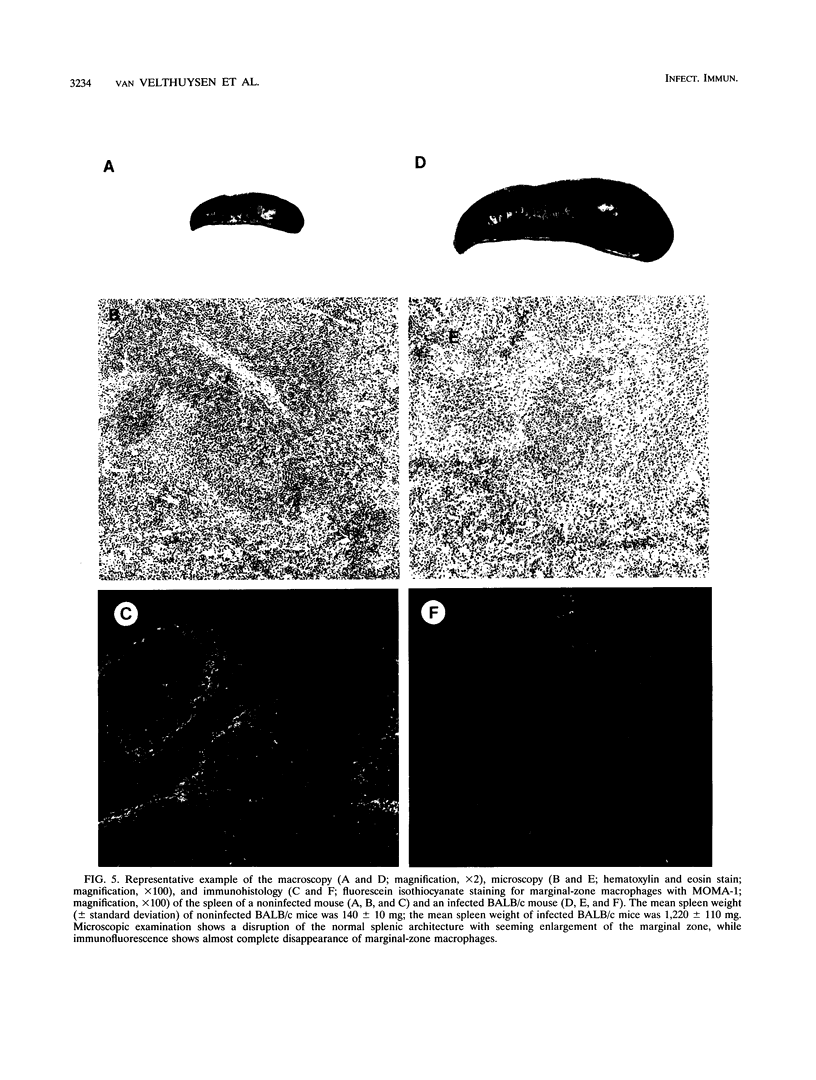
In a previous study, susceptibility for Trypanosoma brucei-related glomerulopathy in mice was shown to be dependent on non-major histocompatibility complex genes. Glomerular disease in this model could not be explained by the production of autoantibodies alone. In order to analyze which part of the defense system, in addition to the B-cell compartment, is involved in the development of this infection-related glomerular disease, groups of athymic (BALB/c rnu/rnu), splenectomized, or macrophage-depleted BALB/c mice were inoculated with T. brucei parasites. Polyclonal B-cell activation, invariably observed in infected BALB/c mice, was absent in BALB/c rnu/rnu mice. Glomerular disease in athymic mice, however, as defined by albuminuria and deposition of immune complexes, was not different from that seen in euthymic infected BALB/c mice. Splenectomy prior to inoculation of parasites led to a decreased incidence of albuminuria in 40% of the animals, whereas splenectomy 21 days after inoculation reduced albuminuria significantly, suggesting a role for spleen cells in the induction of glomerular disease. After macrophage depletion with liposome-encapsulated dichlorodimethylene-diphosphonate, infected BALB/c mice developed significantly higher albuminuria levels for a period up to 2 weeks after depletion. Therefore, it was concluded that the development of T. brucei-related glomerular disease is independent of thymus-matured T cells, while the involvement of macrophages in the development of proteinuria is inhibitory rather than disease inducing. Spleen cells other than thymus-dependent T cells, B cells, and macrophages should be investigated for their role in the pathogenesis of this glomerulopathy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baelde H. J., Bergijk E. C., Bruijn J. A. Isolation and characterization of mouse glomerular basement membrane. J Clin Lab Immunol. 1990 Sep;33(1):17–20. [PubMed] [Google Scholar]
- Barsoum R. S. Schistosomal glomerulopathies. Kidney Int. 1993 Jul;44(1):1–12. doi: 10.1038/ki.1993.205. [DOI] [PubMed] [Google Scholar]
- Bruijn J. A., Oemar B. S., Ehrich J. H., Foidart J. M., Fleuren G. J. Anti-basement membrane glomerulopathy in experimental trypanosomiasis. J Immunol. 1987 Oct 1;139(7):2482–2488. [PubMed] [Google Scholar]
- De Gee A. L., Levine R. F., Mansfield J. M. Genetics of resistance to the African trypanosomes. VI. Heredity of resistance and variable surface glycoprotein-specific immune responses. J Immunol. 1988 Jan 1;140(1):283–288. [PubMed] [Google Scholar]
- Dijkstra C. D., Van Vliet E., Döpp E. A., van der Lelij A. A., Kraal G. Marginal zone macrophages identified by a monoclonal antibody: characterization of immuno- and enzyme-histochemical properties and functional capacities. Immunology. 1985 May;55(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Hendrickse R. G., Adeniyi A. Quartan malarial nephrotic syndrome in children. Kidney Int. 1979 Jul;16(1):64–74. doi: 10.1038/ki.1979.103. [DOI] [PubMed] [Google Scholar]
- Hogendoorn P. C., Bruijn J. A., vd Broek L. J., De Heer E., Foidart J. M., Hoedemaeker P. J., Fleuren G. J. Antibodies to purified renal tubular epithelial antigens contain activity against laminin, fibronectin, and type IV collagen. Lab Invest. 1988 Mar;58(3):278–286. [PubMed] [Google Scholar]
- Kongshavn P. A., Shaw K., Ghadirian E., Ulczak O. Failure to demonstrate a major role for Kupffer cells and radiosensitive leukocytes in immunoglobulin-mediated elimination of Trypanosoma musculi. Infect Immun. 1990 Jun;58(6):1971–1978. doi: 10.1128/iai.58.6.1971-1978.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- Kraal G., Janse M. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 1986 Aug;58(4):665–669. [PMC free article] [PubMed] [Google Scholar]
- Makumyaviri A. M., Sileghem M., Le Ray D., Hamers R., de Baetselier P. Système lymphocytaire et résistance relative de la souris consanguine à Trypanosoma brucei brucei. Ann Inst Pasteur Immunol. 1988 Sep-Oct;139(5):545–556. doi: 10.1016/0769-2625(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Morrison W. I., Murray M., Bovell D. L. Response of the murine lymphoid system to a chronic infection with Trypanosoma congolense. I. The spleen. Lab Invest. 1981 Dec;45(6):547–557. [PubMed] [Google Scholar]
- Morrison W. I., Murray M. The role of humoral immune responses in determining susceptibility of A/J and C57BL/6 mice to infection with Trypanosoma congolense. Parasite Immunol. 1985 Jan;7(1):63–79. doi: 10.1111/j.1365-3024.1985.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Olsson T., Bakhiet M., Höjeberg B., Ljungdahl A., Edlund C., Andersson G., Ekre H. P., Fung-Leung W. P., Mak T., Wigzell H. CD8 is critically involved in lymphocyte activation by a T. brucei brucei-released molecule. Cell. 1993 Mar 12;72(5):715–727. doi: 10.1016/0092-8674(93)90400-k. [DOI] [PubMed] [Google Scholar]
- Orkin R. W., Gehron P., McGoodwin E. B., Martin G. R., Valentine T., Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977 Jan 1;145(1):204–220. doi: 10.1084/jem.145.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Berg R., Martin G. R., Foidart J. M., Robey P. G. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980 May 1;104(1):205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- Savin V. J. Mechanisms of proteinuria in noninflammatory glomerular diseases. Am J Kidney Dis. 1993 Apr;21(4):347–362. doi: 10.1016/s0272-6386(12)80260-5. [DOI] [PubMed] [Google Scholar]
- Seed J. R., Sechelski J. B. African trypanosomes: inheritance of factors involved in resistance. Exp Parasitol. 1989 Jul;69(1):1–8. doi: 10.1016/0014-4894(89)90164-1. [DOI] [PubMed] [Google Scholar]
- Seggie J., Nathoo K., Davies P. G. Association of hepatitis B (HBs) antigenaemia and membranous glomerulonephritis in Zimbabwean children. Nephron. 1984;38(2):115–119. doi: 10.1159/000183291. [DOI] [PubMed] [Google Scholar]
- Sileghem M., Flynn J. N. Suppression of interleukin 2 secretion and interleukin 2 receptor expression during tsetse-transmitted trypanosomiasis in cattle. Eur J Immunol. 1992 Mar;22(3):767–773. doi: 10.1002/eji.1830220321. [DOI] [PubMed] [Google Scholar]
- Sileghem M., Hamers R., De Baetselier P. Experimental Trypanosoma brucei infections selectively suppress both interleukin 2 production and interleukin 2 receptor expression. Eur J Immunol. 1987 Oct;17(10):1417–1421. doi: 10.1002/eji.1830171005. [DOI] [PubMed] [Google Scholar]
- Strauss J., Zilleruelo G., Abitbol C., Montane B., Pardo V. Human immunodeficiency virus nephropathy. Pediatr Nephrol. 1993 Apr;7(2):220–225. doi: 10.1007/BF00864411. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Van Leer E. H., Ronco P., Verroust P., van der Wal A. M., Hoedemaeker P. J., De Heer E. Epitope specificity of anti-gp330 autoantibodies determines the development of proteinuria in active Heymann nephritis. Am J Pathol. 1993 Mar;142(3):821–829. [PMC free article] [PubMed] [Google Scholar]
- Verroust P., Ronco P., Chatelet F. Antigenic targets in membranous glomerulonephritis. Springer Semin Immunopathol. 1987;9(4):341–358. doi: 10.1007/BF00197213. [DOI] [PubMed] [Google Scholar]
- van Rooijen N. Liposome-mediated elimination of macrophages. Res Immunol. 1992 Feb;143(2):215–219. doi: 10.1016/s0923-2494(92)80169-l. [DOI] [PubMed] [Google Scholar]
- van Rooijen N. Macrophages as accessory cells in the in vivo humoral immune response: from processing of particulate antigens to regulation by suppression. Semin Immunol. 1992 Aug;4(4):237–245. [PubMed] [Google Scholar]
- van Velthuysen M. L., Bruijn J. A., van Leer E. H., Fleuren G. J. Pathogenesis of trypanosomiasis-induced glomerulonephritis in mice. Nephrol Dial Transplant. 1992;7(6):507–515. [PubMed] [Google Scholar]
- van Velthuysen M. L., Veninga A., Bruijn J. A., de Heer E., Fleuren G. J. Susceptibility for infection-related glomerulopathy depends on non-MHC genes. Kidney Int. 1993 Mar;43(3):623–629. doi: 10.1038/ki.1993.91. [DOI] [PubMed] [Google Scholar]