Abstract
Oestrogen-induced prolactinomas have been reported in male-to-female (MTF) transgender patients after excessive oestrogen self-administration. Here, two prolactinoma cases after 14 years (case 1) and 30 years (case 2) of relatively low-dose oestrogen treatment are reported. Both resolved after treatment with dopamine agonists. During the first year of oestrogen treatment the patient in case 1 showed a remarkable (7.2-fold) increase in serum prolactin concentration, returning to within the normal range for 13 years until the start of autonomous prolactin secretion. It is hypothesised that this strong first-year prolactin response may be a sign of increased pituitary oestrogen sensitivity. Therefore the patient’s increase in prolactin concentration during the first 18 months was compared to 74 matched control patients from a database, and this increase was found to be significantly greater in the case patient. It is suggested that in MTF patients an excessive first year increase in serum prolactin concentration may identify patients at risk for autonomous prolactin secretion later in life.
BACKGROUND
Oestrogens significantly promote the synthesis and the release of prolactin (PRL) from the pituitary in a dose-dependent and duration-dependent fashion. Oestrogen use in women is widespread, however oestrogen use in men is largely limited to male-to-female (MTF) transgender patients for the induction of feminisation, during which the oestrogen dosages administered are much higher than those used in women. Following oestrogen treatment initiation in MTF transgender patients, a rise in serum prolactin is observed after 4 months of treatment, after which serum prolactin does not significantly change. Highly elevated prolactin levels may be associated with pituitary enlargement.1 To date, four cases of prolactinoma have been reported in MTF patients while using oestrogen dosages far beyond recommended dosages.2–5 This may suggest that prolonged dosage of high oestrogen concentrations may lead to autonomous prolactin secretion. Normal-dose oestrogen treatment in MTF patients has not been associated with development of a prolactinoma.1–3 We present two cases of development of prolactinomas in MTF patients using conventional oestrogen dosages.
CASE PRESENTATION
Case 1
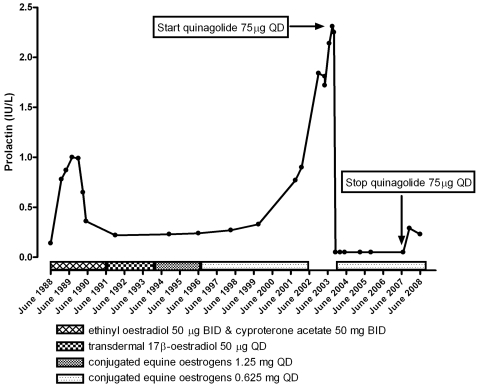
A 36-year-old MTF patient applied for sex reassignment treatment (SRT) in December 1987. The patient had not previously used oestrogens or psychopharmacological medication affecting PRL secretion. Hormonal analysis before cross-sex hormone treatment (CSHT) was unremarkably male. Serum prolactin was 0.14 IU/litre (reference values <0.30 IU/litre). CSHT was initiated in November 1988 with the antiandrogen cyproterone acetate at 50 mg twice a day and the oestrogen ethinyl estradiol at 50 μg twice a day (fig 1). During the course of this period the serum prolactin concentration gradually rose to a maximum of 1.0 IU/litre. While continuing the same dose of ethinyl oestradiol and cyproterone acetate, the serum prolactin concentration returned to the reference range 6 months after it had reached a maximum. In June 1991 upon sex reassignment surgery, including orchiectomy, cyproterone acetate was discontinued. Simultaneously, oestrogen treatment was continued with a much less potent oestrogenic regimen; transdermal patches of 17-β-oestradiol 50 μg per day. However, this caused skin irritation and treatment was changed to oral conjugated equine oestrogens at 1.25 mg daily in October 1993. The patient showed chloasma in July 1995 and the dosage of conjugated equine oestrogen was halved to 0.625 mg daily.
Figure 1.
Case 1 serum prolactin concentration (IU/litre) and cross-sex hormone treatment protocol. During the first 18 months of treatment the serum prolactin rose to a maximum of 1.0 IU/litre, then normalised. After 14 years of treatment autonomous hyperprolactinaemia occurred. This was successfully treated with quinagolide 75 μg daily, after which the serum prolactin concentration returned to within reference levels.
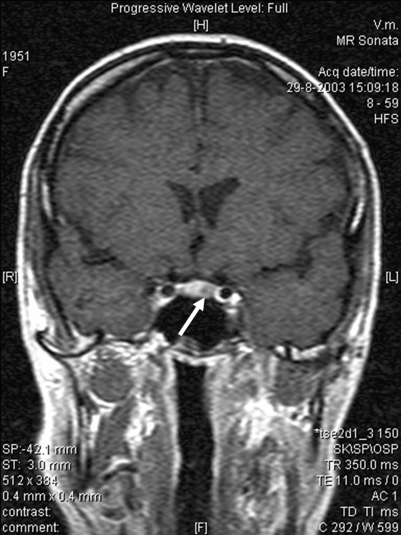
Serum prolactin levels were unremarkable at annual visits until January 2003, when a serum prolactin concentration of 1.84 IU/litre was measured (fig 1). The patient had no clinical symptoms of a prolactinoma. As part of follow-up routine investigations, after cessation of oestrogen administration, serum prolactin was found to have increased to a maximum level of 2.3 IU/litre, suggesting autonomous prolactin secretion. MRI revealed a focal hypointense lesion of the pituitary gland, 8 mm in diameter (fig 2). There were no signs of compression of the optic chiasm. Treatment with the dopamine agonist quinagolide, 75 μg daily, reduced serum PRL levels to undetectable concentrations.
Figure 2.
Contrast-enhanced coronal T1-weighted MRI. The left side of the pituitary gland shows a focal hypointense lesion (arrow) with a diameter of 8 mm.
Case 2
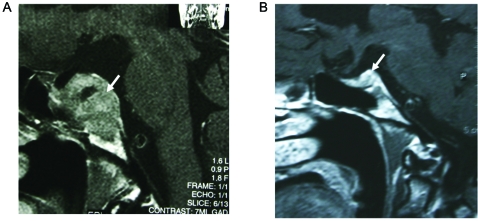
A 69-year-old MTF patient was admitted with sudden onset of double vision. She had been taking ethinyloestradiol 50 µg daily for 30 years. On examination she had a right-sided third nerve palsy with no visual field defects. Her serum prolactin level was 40 IU/litre (reference values <0.30 IU/litre). Gonadotropin levels were suppressed and T4 levels were low. An MRI scan of the pituitary gland revealed a large pituitary adenoma that had invaded the cavernous sinus on the right hand side and abutted on the chiasm (fig 3). Immediately, cabergoline 0.25 mg twice weekly and levothyroxine 50 µg daily were given. After 10 days serum prolactin had declined to 6.47 IU/litre. Her third nerve palsy resolved, and an MRI scan after 4 months of treatment showed an appreciable shrinkage with no invasion of the chiasmatic cistern and the right cavernous sinus (fig 3). Over time serum prolactin levels normalised. She refused to stop oestrogen treatment but, with cabergoline treatment, serum prolactin levels returned to normal and have remained within the normal range for the past 8 years. The tumour has never increased in size again.
Figure 3.
A sagittal MRI scan (with gadolinium) showing an oestrogen-induced prolactinoma extending into the suprasellar cistern (A) and regression of the tumour after 4 months of cabergoline treatment (arrow) (B). Note also that the tumour is not abutting on the chiasm.
OUTCOME AND FOLLOW-UP
In the first case serum prolactin concentration remained undetectable until the cessation of the quinagolide treatment in August 2007, after which prolactin levels remained within the reference range during follow-up (last performed in June 2008), 20 years after initiation of cross-sex hormone treatment. The second case has been in remission since the year 2000. Cabergoline and oestrogen treatment have been stopped since May 2008 and serum prolactin levels have remained normal.
DISCUSSION
Even though some MTF patients self-administer oestrogen dosages far beyond the recommended dosage range, prolactinomas are a rare occurrence in this population.1–3 We, among others, have previously described MTF patients who have developed a prolactinoma following cross-sex hormone administration with oestrogens.3–6 The cases presented in this report differ from previously reported cases. Firstly, these patients have not used oestrogen dosages exceeding recommended dosages. The absence of luteinising hormone (LH) and follicle-stimulating hormone (FSH) suppression in the first case throughout the observed period supports this contention. Secondly, in the cases so far reported the prolactinomas emerged after a period of oestrogen exposure of 9 months to 8 years, while in the presented cases the prolactinoma became manifest after 14 and 30 years, respectively, of relatively low-dose oestrogen exposure. What distinguished the first case from other similarly oestrogen-treated transgender patients was the exaggerated response of prolactin to oestrogen treatment during the first 18 months, which was significantly higher compared to controls matched for patients matched for age (30 to 40), smoking status (non-smoker), oestrogen use prior to treatment (no), treatment regime (cyproterone acetate 50 mg twice a day and the oestrogen ethinyl estradiol 50 μg twice a day) (table 1).
Table 1.
Absolute (IU/litre) and relative (fold) increase in serum prolactin levels within the first 18 months of cross-sex hormone treatment with 50 μg ethinyl oestradiol and 50 mg cyproterone acetate twice a day
| Case | Controls (n=74) | |
| Absolute prolactin increase (IU/litre) | 0.86* | 0.37 (0.30 to 0.44) |
| Relative prolactin increase (fold) | 7.2* | 3.2 (2.7 to 3.7) |
Controls were matched for ethnicity, smoking status, cross-sex hormone treatment and duration of treatment. Data reported as mean (95% CI).
*p<0.05
In an earlier report by our group we found substantial individual differences in prolactin concentrations in patients on a standard endocrine treatment regimen (50 mg cyproterone acetate twice a day combined with 50 μg ethinyl oestradiol twice a day)1 but the initial response in case 1 to conventional treatment exceeded far those of others. Pretreatment plasma oestradiol concentrations did not differ between this case patient and the control group (table 2). However, before the start of the cross-sex hormonal treatment the case patient showed a slightly lower serum testosterone concentration combined with a lower luteinising hormone concentration, which, in theory, may have indicated increased pituitary sensitivity to oestrogen negative feedback, causing a serum testosterone reduction. After orchiectomy the patients did not show signs and symptoms of oestrogen deficiency, despite the very low oestrogen doses used as hormone replacement therapy, which may be another indication of high oestrogen sensitivity.
Table 2.
Serum prolactin, luteinising hormone, follicle stimulating hormone, 17-β-oestradiol and testosterone concentration prior and after 18 months of 50 μg ethinyl oestradiol and 50 mg cyproterone acetate twice a day administration in the case patient and 74 matched controls
| Baseline | 18 months | |||
| Case | Controls (n=74) | Case | Controls (n=74) | |
| Prolactin (IU/litre) | 0.14 | 0.20 (0.17 to 0.23) | 1.00 | 0.57 (0.48 to 0.66) |
| Luteinising hormone (IU/litre) | 1.6* | 3.0 (2.5 to 3.5) | 0.3 | 0.4 (0.1 to 0.8) |
| Follicle stimulating hormone (IU/litre) | 4.5 | 3.8 (2.9 to 4.7) | 1.0 | 1.7 (0.1 to 3.5) |
| 17-β-oestradiol (pmol/litre) | 92.0 | 83.1 (73.6 to 92.7) | 43.0 | 33.8 (24.6 to 43.0) |
| Testosterone (nmol/litre) | 15.0* | 20.3 (17.6 to 23.1) | 1.0 | 1.3 (0.3 to 2.2) |
Data reported as mean (95% CI).
*p<0.05.
Sex differences in circulating oestrogens may explain the higher basal serum prolactin concentrations and the higher incidence of prolactinomas in women than in men, through effects on the hypothalamus and pituitary.7 Oestrogen receptor α (ERα) and oestrogen receptor β (ERβ) are present in lactotrophic cells,8 and receptor-mediated and (rapid) non-genomic mechanisms may mediate proliferatory effects of oestrogens on normal lactotrophic cells.9 In rats, oestrogen administration can induce prolactinomas10,11 but these regress on oestrogen withdrawal.12 In ERα-deficient mice there is a decline in pituitary prolactin mRNA levels.7 Besides a direct stimulation of prolactin gene transcription, several other oestrogen-responsive genes are thought to be involved in regulating pituitary proliferation (eg, the hypothalamic neurohormone dopamine, cytokines, and autocrine and paracrine growth factors).13
The hypothalamic hormone dopamine normally inhibits prolactin secretion. Loss of dopaminergic tonus or pituitary resistance to the actions of dopamine has been proposed as a causal factor in the development of lactotroph cell hyperplasia or prolactinomas, although this remains disputed.9 Dopaminergic action on the lactotrophic cells is mediated by the dopamine type 2 receptor (DR2). In DR2-deficient mice prolactinomas develop after prolonged lactotrophic hyperplasia.14 In mice, oestrogen downregulates pituitary dopamine receptors.15 Compared to similarly oestrogen-treated transgender patients our patient showed an initial hyper-response of serum prolactin to oestrogen exposure, which (in theory) could be interpreted as a downregulation of DR2 resulting in diminished dopaminergic inhibition of prolactin secretion, which, over time, may have resulted in the development of a prolactinoma. In humans decreased expression of DR2 corresponds clinically to a low response of prolactinomas to dopaminergic drug treatment.9 This was not the case in our patient, as treatment with the dopamine agonist quinagolide rapidly reduced serum prolactin concentrations to non-detectable levels.
We report here the development of prolactinomas in two MTF patients after 14 and 30 years, respectively, of oestrogen administration using a conventional oestrogen dosage. Both cases might represent occult prolactinoma formation.12 A case report of course does not have the power to prove a causal relationship between the emergence of a prolactinoma and previous oestrogen exposure. However, as our finding of an initial increase of serum prolactin is consistent with the hypothesised high oestrogen sensitivity in this patient, we advise continued long-term prolactin monitoring in MTF patients, especially those with an excessive prolactin response after treatment initiation.16
LEARNING POINTS
Prolactinomas may occur as a result of oestrogen misuse in male-to-female (MTF) transgender patients.
Two case reports attest to the possibility of developing prolactinomas after long-term use of conventional oestrogen use.
An initial steep increase in serum prolactin concentrations during the first 18 months of treatment may be an indication of increased oestrogen sensitivity.
MTF patients showing this steep increase may be at higher risk of developing autonomous prolactin secretion later in life.
Serum prolactin concentrations should be monitored annually in MTF transgender patients, even after a long-term, apparently inconspicuous treatment period.
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Asscheman H, Gooren LJ, Assies J, et al. Prolactin levels and pituitary enlargement in hormone-treated male-to-female transsexuals. Clin Endocrinol (Oxf) 1988; 28: 583–8 [DOI] [PubMed] [Google Scholar]
- 2.Levy A, Crown A, Reid R. Endocrine intervention for transsexuals. Clin Endocrinol 2003; 59: 409–18 [DOI] [PubMed] [Google Scholar]
- 3.Gooren LJ, Assies J, Asscheman H, et al. Estrogen-induced prolactinoma in a man. J Clin Endocrinol Metab 1988; 66: 444–6 [DOI] [PubMed] [Google Scholar]
- 4.Futterweit W. Endocrine therapy of transsexualism and potential complications of long-term treatment. Arch Sex Behav 1998; 27: 209–26 [DOI] [PubMed] [Google Scholar]
- 5.Kovacs K, Stefaneanu L, Ezzat S, et al. Prolactin-producing pituitary adenoma in a male-to-female transsexual patient with protracted estrogen administration. A morphologic study. Arch Pathol Lab Med 1994; 118: 562–5 [PubMed] [Google Scholar]
- 6.Serri O, Noiseux D, Robert F, et al. Lactotroph hyperplasia in an estrogen treated male-to-female transsexual patient. J Clin Endocrinol Metab 1996; 81: 3177–9 [DOI] [PubMed] [Google Scholar]
- 7.Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocrine Rev 2001; 22: 724–63 [DOI] [PubMed] [Google Scholar]
- 8.Nishihara E, Nagayama Y, Inoue S, et al. Ontogenetic changes in the expression of estrogen receptor alpha and beta in rat pituitary gland detected by immunohistochemistry. Endocrinology 2000; 141: 615–20 [DOI] [PubMed] [Google Scholar]
- 9.Velkeniers B. From prolactin cell to prolactinoma. Verh K Acad Geneeskd Belg 2001; 63: 561–73 [PubMed] [Google Scholar]
- 10.Spady TJ, Harvell DM, Snyder MC, et al. Estrogen-induced tumorigenesis in the Copenhagen rat: disparate susceptibilities to development of prolactin-producing pituitary tumors and mammary carcinomas. Cancer Lett 1998; 124: 95–103 [DOI] [PubMed] [Google Scholar]
- 11.Spady TJ, McComb RD, Shull JD. Estrogen action in the regulation of cell proliferation, cell survival, and tumorigenesis in the rat anterior pituitary gland. Endocrine 1999; 11: 217–33 [DOI] [PubMed] [Google Scholar]
- 12.Teip CS. The regression of oestradiol-induced pituitary tumours in the rat. J Pathol 1983; 141: 29–40 [DOI] [PubMed] [Google Scholar]
- 13.Ishida M, Takahashi W, Itoh S, et al. Estrogen actions on lactotroph proliferation are independent of a paracrine interaction with other pituitary cell types: a study using lactotroph-enriched cells. Endocrinology 2007; 148: 3131–9 [DOI] [PubMed] [Google Scholar]
- 14.Asa SL, Kelly MA, Grandy DK, et al. Pituitary lactotroph adenomas develop after prolonged lactotroph hyperplasia in dopamine D2 receptor-deficient mice. Endocrinology 1999; 140: 5348–55 [DOI] [PubMed] [Google Scholar]
- 15.Kochman K, Joseph JA, Blackman MR, et al. Impaired down-regulation of pituitary dopamine receptors by estradiol in aged rats. Proc Soc Exp Biol Med 1989; 192: 23–6 [DOI] [PubMed] [Google Scholar]
- 16.Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2009. In press. [DOI] [PubMed] [Google Scholar]