Abstract
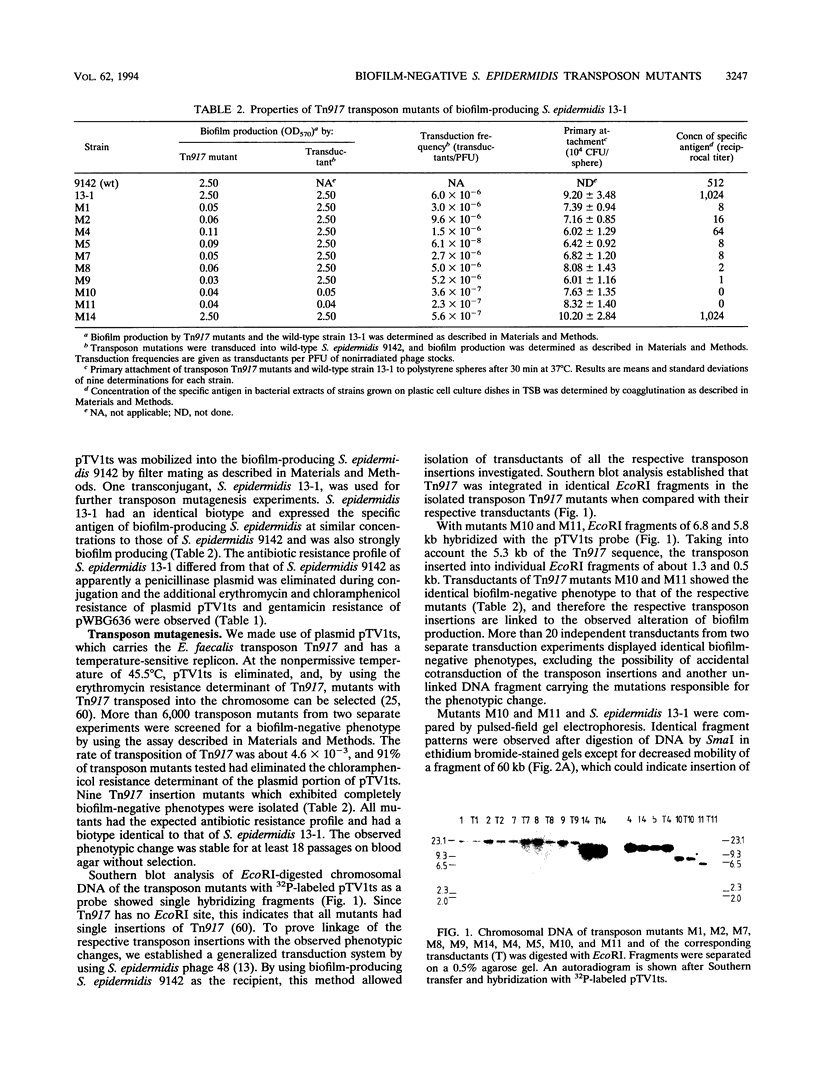
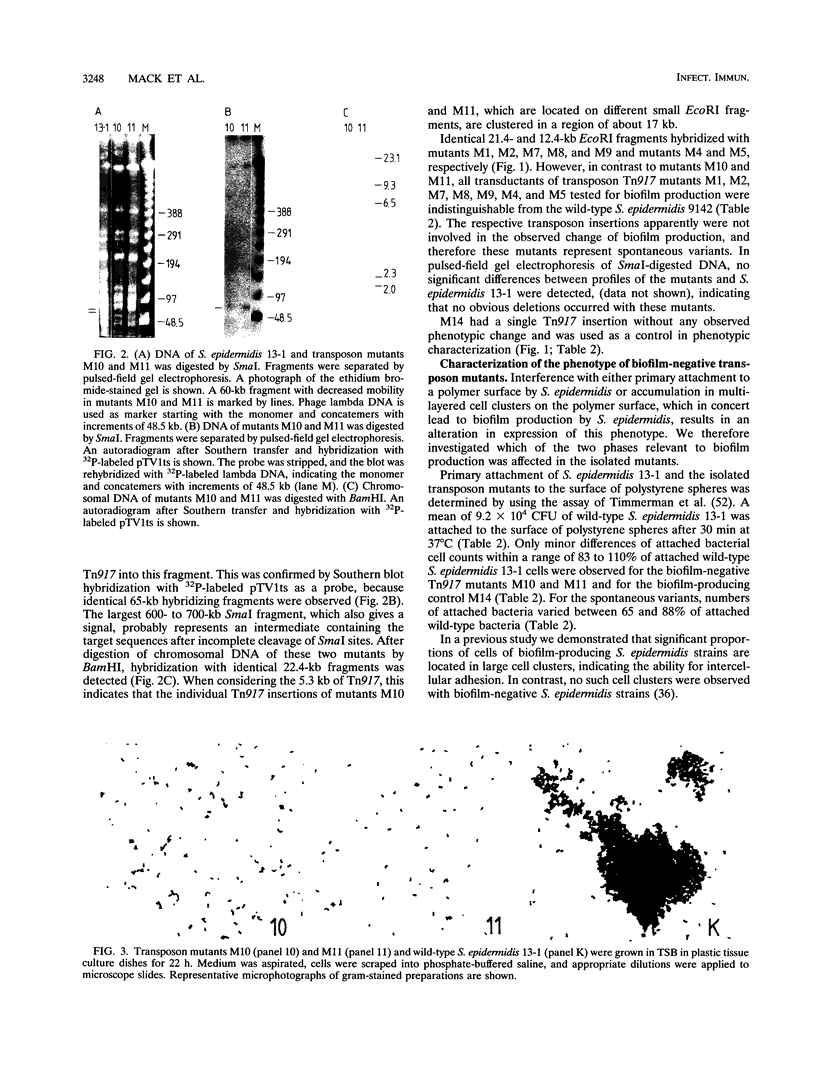
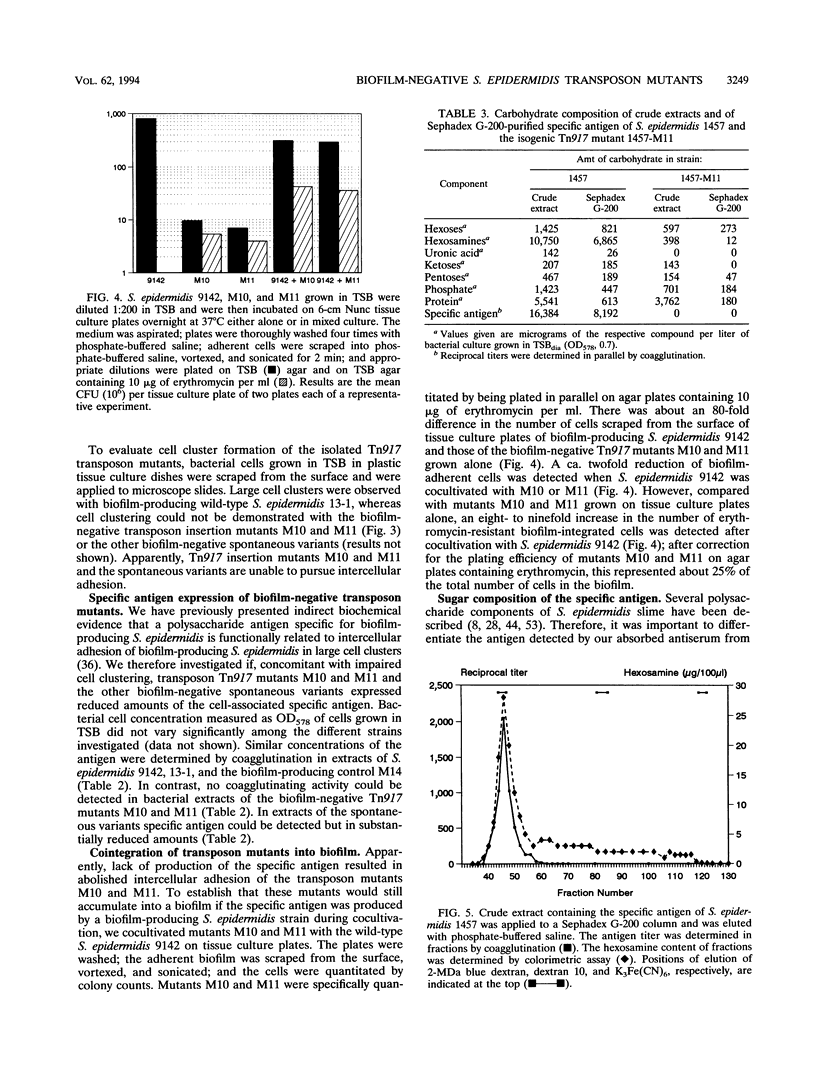
The primary attachment to polymer surfaces followed by accumulation in multilayered cell clusters leads to production of Staphylococcus epidermidis biofilms, which are thought to contribute to virulence in biomaterial-related infections. We isolated Tn917 transposon mutants of biofilm-producing S. epidermidis 13-1, which were completely biofilm negative. In pulsed-field gel electrophoresis no obvious deletions of the mutants were noted. The Tn917 insertions of mutants M10 and M11 were located on different EcoRI fragments but on identical 60-kb SmaI and 17-kb BamHI chromosomal fragments. Linkage of transposon insertions of mutants M10 and M11 with the altered phenotype was demonstrated by phage transduction, whereas the several other mutants apparently represented spontaneous variants. In a primary attachment assay with polystyrene spheres, no significant difference between any of the mutants and the wild type could be detected. Cell clustering as an indication of intercellular adhesion, which is a prerequisite for accumulation in multilayered cell clusters, was not detected with any mutant. These results demonstrate that the mutants were impaired in the accumulative phase of biofilm production. Mutants M10 and M11 did not produce detectable amounts of a specific polysaccharide antigen (D. Mack, N. Siemssen, and R. Laufs, Infect. Immun. 60:2048-2057, 1992), whereas substantially reduced amounts of antigen were produced by the spontaneous variants. Hexosamine was determined as the major specific component of the antigen enriched by gel filtration of biofilm-producing S. epidermidis 1457 because almost no hexosamine was detected in material prepared from the isogenic biofilm-negative transductant 1457-M11, which differentiates the antigen from other S. epidermidis polysaccharide components. Our results provide direct genetic evidence for a function of the antigen in the accumulative phase of biofilm production by S. epidermidis by mediating intercellular adhesion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayston R., Rodgers J. Production of extra-cellular slime by Staphylococcus epidermidis during stationary phase of growth: its association with adherence to implantable devices. J Clin Pathol. 1990 Oct;43(10):866–870. doi: 10.1136/jcp.43.10.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Madison B. M., Parisi J. T., Abraham S. N., Hasty D. L., Lowrance J. H., Josephs J. A., Simpson W. A. Colonial morphology of staphylococci on Memphis agar: phase variation of slime production, resistance to beta-lactam antibiotics, and virulence. J Infect Dis. 1990 Jun;161(6):1153–1169. doi: 10.1093/infdis/161.6.1153. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Simpson W. A. Phenotypic variation of Staphylococcus epidermidis slime production in vitro and in vivo. Infect Immun. 1987 Dec;55(12):2870–2877. doi: 10.1128/iai.55.12.2870-2877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Barker L. P., Mawhinney T. P., Baddour L. M., Simpson W. A. Identification of an antigenic marker of slime production for Staphylococcus epidermidis. Infect Immun. 1990 Sep;58(9):2906–2911. doi: 10.1128/iai.58.9.2906-2911.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Younger J. J., Baddour L. M., Barrett F. F., Melton D. M., Beachey E. H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985 Dec;22(6):996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Davenport D. S., Massanari R. M., Pfaller M. A., Bale M. J., Streed S. A., Hierholzer W. J., Jr Usefulness of a test for slime production as a marker for clinically significant infections with coagulase-negative staphylococci. J Infect Dis. 1986 Feb;153(2):332–339. doi: 10.1093/infdis/153.2.332. [DOI] [PubMed] [Google Scholar]
- Dean B. A., Williams R. E., Hall F., Corse J. Phage typing of coagulase-negative staphylococci and micrococci. J Hyg (Lond) 1973 Jun;71(2):261–270. doi: 10.1017/s0022172400022737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighton M. A., Balkau B. Adherence measured by microtiter assay as a virulence marker for Staphylococcus epidermidis infections. J Clin Microbiol. 1990 Nov;28(11):2442–2447. doi: 10.1128/jcm.28.11.2442-2447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Mitoma F., Harding G. K., Hoban D. J., Roberts R. S., Low D. E. Clinical significance of a test for slime production in ventriculoperitoneal shunt infections caused by coagulase-negative staphylococci. J Infect Dis. 1987 Oct;156(4):555–560. doi: 10.1093/infdis/156.4.555. [DOI] [PubMed] [Google Scholar]
- Drewry D. T., Galbraith L., Wilkinson B. J., Wilkinson S. G. Staphylococcal slime: a cautionary tale. J Clin Microbiol. 1990 Jun;28(6):1292–1296. doi: 10.1128/jcm.28.6.1292-1296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne W. M., Jr, Nelson D. B., Chusid M. J. Epidemiologic markers of pediatric infections caused by coagulase-negative staphylococci. Pediatr Infect Dis J. 1987 Nov;6(11):1031–1035. [PubMed] [Google Scholar]
- Espersen F., Wilkinson B. J., Gahrn-Hansen B., Thamdrup Rosdahl V., Clemmensen I. Attachment of staphylococci to silicone catheters in vitro. APMIS. 1990 May;98(5):471–478. [PubMed] [Google Scholar]
- Foster T. J. Potential for vaccination against infections caused by Staphylococcus aureus. Vaccine. 1991 Apr;9(4):221–227. doi: 10.1016/0264-410x(91)90103-d. [DOI] [PubMed] [Google Scholar]
- Franson T. R., Sheth N. K., Rose H. D., Sohnle P. G. Scanning electron microscopy of bacteria adherent to intravascular catheters. J Clin Microbiol. 1984 Sep;20(3):500–505. doi: 10.1128/jcm.20.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann D. A., Pier G. B. Pathogenesis of infections related to intravascular catheterization. Clin Microbiol Rev. 1993 Apr;6(2):176–192. doi: 10.1128/cmr.6.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter L., Koenig O., Laufs R. Transposon mutagenesis in Staphylococcus epidermidis using the Enterococcus faecalis transposon Tn917. FEMS Microbiol Lett. 1991 Aug 1;66(2):215–218. doi: 10.1016/0378-1097(91)90335-8. [DOI] [PubMed] [Google Scholar]
- Grüter L., Feucht H., Mempel M., Laufs R. Construction of a slime negative transposon mutant in Staphylococcus epidermidis using the Enterococcus faecalis transposon Tn917. Microbiol Immunol. 1993;37(1):35–40. doi: 10.1111/j.1348-0421.1993.tb03176.x. [DOI] [PubMed] [Google Scholar]
- Hogt A. H., Dankert J., Hulstaert C. E., Feijen J. Cell surface characteristics of coagulase-negative staphylococci and their adherence to fluorinated poly(ethylenepropylene). Infect Immun. 1986 Jan;51(1):294–301. doi: 10.1128/iai.51.1.294-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Hastings J. G., White P. J. Comparison of cell-wall teichoic acid with high-molecular-weight extracellular slime material from Staphylococcus epidermidis. J Med Microbiol. 1992 Dec;37(6):368–375. doi: 10.1099/00222615-37-6-368. [DOI] [PubMed] [Google Scholar]
- Hussain M., Hastings J. G., White P. J. Isolation and composition of the extracellular slime made by coagulase-negative staphylococci in a chemically defined medium. J Infect Dis. 1991 Mar;163(3):534–541. doi: 10.1093/infdis/163.3.534. [DOI] [PubMed] [Google Scholar]
- Hussain M., Wilcox M. H., White P. J., Faulkner M. K., Spencer R. C. Importance of medium and atmosphere type to both slime production and adherence by coagulase-negative staphylococci. J Hosp Infect. 1992 Mar;20(3):173–184. doi: 10.1016/0195-6701(92)90085-z. [DOI] [PubMed] [Google Scholar]
- Hussain M., Wilcox M. H., White P. J. The slime of coagulase-negative staphylococci: biochemistry and relation to adherence. FEMS Microbiol Rev. 1993 Apr;10(3-4):191–207. doi: 10.1111/j.1574-6968.1993.tb05867.x. [DOI] [PubMed] [Google Scholar]
- Ishak M. A., Gröschel D. H., Mandell G. L., Wenzel R. P. Association of slime with pathogenicity of coagulase-negative staphylococci causing nosocomial septicemia. J Clin Microbiol. 1985 Dec;22(6):1025–1029. doi: 10.1128/jcm.22.6.1025-1029.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser F. H., Wüst J., Corrodi P. Transduction and elimination of resistance determinants in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Sep;2(3):217–223. doi: 10.1128/aac.2.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwicka A., Uhlenbruck G., Peters G., Seng P. N., Gray E. D., Jeljaszewicz J., Pulverer G. Investigation on extracellular slime substance produced by Staphylococcus epidermidis. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Dec;258(2-3):256–267. doi: 10.1016/s0176-6724(84)80043-7. [DOI] [PubMed] [Google Scholar]
- Mack D., Siemssen N., Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992 May;60(5):2048–2057. doi: 10.1128/iai.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J Clin Microbiol. 1984 May;19(5):687–693. doi: 10.1128/jcm.19.5.687-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Pfaller M. A., Wenzel R. P. Coagulase-negative staphylococcal bacteremia. Mortality and hospital stay. Ann Intern Med. 1989 Jan 1;110(1):9–16. doi: 10.7326/0003-4819-110-1-9. [DOI] [PubMed] [Google Scholar]
- Muller E., Hübner J., Gutierrez N., Takeda S., Goldmann D. A., Pier G. B. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect Immun. 1993 Feb;61(2):551–558. doi: 10.1128/iai.61.2.551-558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller E., Takeda S., Shiro H., Goldmann D., Pier G. B. Occurrence of capsular polysaccharide/adhesin among clinical isolates of coagulase-negative staphylococci. J Infect Dis. 1993 Nov;168(5):1211–1218. doi: 10.1093/infdis/168.5.1211. [DOI] [PubMed] [Google Scholar]
- Pascual A., Fleer A., Westerdaal N. A., Verhoef J. Modulation of adherence of coagulase-negative staphylococci to Teflon catheters in vitro. Eur J Clin Microbiol. 1986 Oct;5(5):518–522. doi: 10.1007/BF02017694. [DOI] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Microbial colonization of prosthetic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):293–299. [PubMed] [Google Scholar]
- Pfaller M. A., Herwaldt L. A. Laboratory, clinical, and epidemiological aspects of coagulase-negative staphylococci. Clin Microbiol Rev. 1988 Jul;1(3):281–299. doi: 10.1128/cmr.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosdahl V. T., Gahrn-Hansen B., Møller J. K., Kjaeldgaard P. Phage-typing of coagulase-negative staphylococci. Factors influencing typability. APMIS. 1990 Apr;98(4):299–304. doi: 10.1111/j.1699-0463.1990.tb01036.x. [DOI] [PubMed] [Google Scholar]
- SWANSTROM M., ADAMS M. H. Agar layer method for production of high titer phage stocks. Proc Soc Exp Biol Med. 1951 Nov;78(2):372–375. doi: 10.3181/00379727-78-19076. [DOI] [PubMed] [Google Scholar]
- Schmitt D. D., Bandyk D. F., Pequet A. J., Malangoni M. A., Towne J. B. Mucin production by Staphylococcus epidermidis. A virulence factor promoting adherence to vascular grafts. Arch Surg. 1986 Jan;121(1):89–95. doi: 10.1001/archsurg.1986.01400010103013. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf A., Karch H., Schmidt H., Lenz W., Heesemann J. Phenotypical and genotypical characterization of epidemic clumping factor-negative, oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993 Sep;31(9):2281–2285. doi: 10.1128/jcm.31.9.2281-2285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Gilkerson E. Quantitation of glycosaminoglycan hexosamine using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Anal Biochem. 1979 Oct 1;98(2):478–480. doi: 10.1016/0003-2697(79)90170-2. [DOI] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Archer G. L. Mobilization of recombinant plasmids from Staphylococcus aureus into coagulase negative Staphylococcus species. Plasmid. 1992 Mar;27(2):164–168. doi: 10.1016/0147-619x(92)90017-5. [DOI] [PubMed] [Google Scholar]
- Timmerman C. P., Fleer A., Besnier J. M., De Graaf L., Cremers F., Verhoef J. Characterization of a proteinaceous adhesin of Staphylococcus epidermidis which mediates attachment to polystyrene. Infect Immun. 1991 Nov;59(11):4187–4192. doi: 10.1128/iai.59.11.4187-4192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M., Yamashita N., Goldmann D. A., Pier G. B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988 Apr;157(4):713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Bolton S., Ashdown N., Grubb W. B. Transfer of plasmid-borne aminoglycoside-resistance determinants in staphylococci. J Med Microbiol. 1985 Oct;20(2):169–185. doi: 10.1099/00222615-20-2-169. [DOI] [PubMed] [Google Scholar]
- Udo E. E., Grubb W. B. A new class of conjugative plasmid in Staphylococcus aureus. J Med Microbiol. 1990 Mar;31(3):207–212. doi: 10.1099/00222615-31-3-207. [DOI] [PubMed] [Google Scholar]
- Udo E. E., Grubb W. B. Conjugal transfer of plasmid pWBG637 from Staphylococcus aureus to Staphylococcus epidermidis and Streptococcus faecalis. FEMS Microbiol Lett. 1990 Oct;60(1-2):183–187. doi: 10.1016/0378-1097(90)90369-2. [DOI] [PubMed] [Google Scholar]
- Vaudaux P., Pittet D., Haeberli A., Huggler E., Nydegger U. E., Lew D. P., Waldvogel F. A. Host factors selectively increase staphylococcal adherence on inserted catheters: a role for fibronectin and fibrinogen or fibrin. J Infect Dis. 1989 Nov;160(5):865–875. doi: 10.1093/infdis/160.5.865. [DOI] [PubMed] [Google Scholar]
- Younger J. J., Christensen G. D., Bartley D. L., Simmons J. C., Barrett F. F. Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis. 1987 Oct;156(4):548–554. doi: 10.1093/infdis/156.4.548. [DOI] [PubMed] [Google Scholar]
- de Saxe M. J., Notley C. M. Experiences with the typing of coagulase-negative staphylococci and micrococci. Zentralbl Bakteriol Orig A. 1978 Jul;241(1):46–59. [PubMed] [Google Scholar]






