Abstract
The G2019S leucine-rich repeat kinase 2 gene (LRRK2) mutation has been identified in a significant proportion of familial and sporadic cases of Parkinson’s disease (PD). Until now, information on the neuropathological changes associated with the G2019S LRRK2 mutation has been sparse. We report a 77-year-old patient who presented with a 14 year history of PD but, unexpectedly, histopathological examination disclosed mild neuronal loss in the substantia nigra without α-synuclein, tau or ubiquitin cytoplasmic inclusions. A G2019S LRRK2 mutation was eventually detected. The present case confirms that clinical PD caused by G2019S mutations can be associated with non-specific nigral degeneration without Lewy
BACKGROUND
Recently, pathogenic mutations in a novel gene, the leucine-rich repeat kinase 2 gene (LRRK2), have been identified in a significant proportion of patients with autosomal dominant forms of Parkinson’s disease (PD).1,2 Several LRRK2 mutations have been described, although until now only five, R1441C, R1441G, Y1699C, G2019S and I2020T, are considered definitely pathogenic.3 G2019S is the most common and may account for approximately 5–6% of familial and 1–3% of sporadic PD cases.4–6 The clinical features and response to levodopa treatment in patients with αLRRK2 mutations seem to be indistinguishable from classic PD.3–6
Although information is sparse, the neuropathological changes in patients with PD associated with LRRK2 mutations seems to be heterogeneous.2 In some cases, changes are consistent with classic PD, with neuronal loss in the substantia nigra (SN) and α-synuclein positive Lewy bodies (LB). However, in other cases, pathological findings vary and include the presence of non-specific neuronal loss with ubiquitin reactive cytoplasmic and nuclear inclusions, tau pathology reminiscent of progressive supranuclear palsy or pure nigral degeneration without specific α-synuclein, tau or ubiquitin inclusions.2 We report a patient with a G2019S LRRK2 mutation with a clinical picture compatible with PD, but histopathological examination disclosed non-specific nigral degeneration in the SN without α-synuclein, tau or ubiquitin cytoplasmic inclusions.
CASE PRESENTATION
A 63-year-old woman presented with a 2 year history of rest tremor and slowness in her left limbs. She had a long history of diabetes mellitus type 2 and arterial hypertension. Although her family history for PD was negative, the patient explained that her mother and maternal uncle had suffered upper limb tremor at an older age. Neurological examination disclosed mild rest tremor, bradykinesia and rigidity in her left limbs. Her speech, face, ocular motility, gait and postural stability were normal. Tremor dominant PD, Hoehn and Yahr (H&Y) stage I, was diagnosed and levodopa treatment, 300 mg daily, was started with an excellent response.
Over the next 10 years, PD slightly progressed. Mild speech disturbance and facial hypomimia appeared. Rest tremor, bradykinesia and rigidity in the left limbs slowly worsened, and treatment with pramipexole 0.7 mg three times daily was added. Evident involvement of the right limbs occurred during the ninth year of her illness (H&Y stage II) but PD was still mild and asymmetric. After the 10th year of illness, shuffling and freezing of gait with postural instability developed (H&Y stage III). Levodopa was increased to 600 mg daily and moderate but bothersome coreiform dyskinesias in her left foot and abnormal posture in her trunk, which was bent to the right, appeared. Over the next 3 years her gait abnormalities worsened with the presence of frequent falls. Mild motor fluctuations of the wearing-off type also occurred. In the 13th year of illness, the patient needed assistance with walking (H&Y stage IV).
In the 14th year of her illness, the patient developed confusional syndrome with seizures related to heart failure–hypertensive cardiopathy and renal insufficiency–diabetic nephropathy. A brain MRI performed at that time disclosed severe periventricular leukoaraiosis with subcortical lacunar infarcts with mild rarefaction of the pons white matter. The confusional state and seizures were complicated by pneumonia, which led to death. Signs of cognitive impairment, hallucinations, delusions or atypical signs for PD never developed during the course of her illness.
INVESTIGATIONS
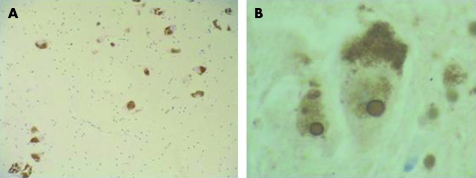
Neuropathological examination was carried out following a well established protocol at the Universitat Barcelona-Clínic Brain Bank using haematoxylin-eosin and luxol fast blue–Klüver Barrera staining, and immunohistochemistry with specific antibodies to BA4 amyloid, phosphorylated tau, ubiquitin, α-synuclein, CD20 and CD3, neurofilaments and αß-crystallin. The examination revealed mild depigmentation of the SN and multiple subcortical and basal ganglia lacunar infarcts. Neuronal loss in the SN was mild, involving both pars compacta and reticulata, with free extraneuronal melanin and prominent eosinophilic ubiquitin positive intanuclear inclusions compatible with Marinesco bodies (fig 1). Mild neuronal loss with abundant Marinesco bodies was also observed in the locus coeruleus. Neuronal loss was not detected in other brain structures. α-Synuclein immunohistochemistry did not disclose LB or Lewy neurites in the SN or other brain areas. Isolated tau positive neurofibrillary tangles and neuropil threads were observed in the hippocampus, transentorhinal cortex, locus coeruleus, nucleus basalis of Meynert and brainstem periaqueductal grey matter, but amyloid plaques were absent. A heterozygous G2019S LRRK2 mutation was detected using the methods previously described from DNA obtained from frozen brain tissue.6
Figure 1. α-Synuclein immunohistochemistry (×100 objective) showing mild neuronal loss and gliosis of the substantia nigra without Lewy bodies or Lewy neurites (A).
Ubiquitin immunohistochemistry (×1000 objective) showing prominent nuclear inclusions in neurons of the substantia nigra compatible with Marinesco bodies (B).
DISCUSSION
Our patient presented with a progressive PD syndrome which started unilaterally with rest tremor, and showed a good response to levodopa with development of classic treatment induced motor complications without the presence of atypical features for PD. The patient fulfilled the accepted criteria used for a clinical diagnosis of PD but, unexpectedly, the histopathology studies disclosed only mild neuronal loss in the SN without α-synuclein positive LB or Lewy neurites.7
Cases of parkinsonism associated with LRRK2 mutations with histopathological findings similar to those reported here led us to search for a mutation in this gene. Pure nigral degeneration without α-synuclein positive LB and Lewy neurites was described as the underlying neuropathological substrate in the first PD family linked to the PARK8 locus, the Sagamihara kindred, that later was found to carry the missense mutation I2020T in the LRRK2 gene.8,9 Non-specific nigral degeneration has also been reported in patients with other LRRK2 mutations. In one subject (family D; Western Nebraska; mutation R1441C), the SN showed marked neuronal loss without α-synuclein inclusions but ubiquitin positive inclusions were observed whereas two other individuals of this kindred presented with LB disease and another had tau positive inclusions.2 In three subjects carrying the mutation Y1699C, non-specific SN degeneration with ubiquitin positive neuronal inclusions was observed in two cases whereas LB formation was present in one case.2,10
To date, the neuropathology associated with the commonest LRRK2 mutation, G2019S, has been examined in only 14 cases.5,11,12 In 13 cases, LB and Lewy neurites were found and, therefore, some authors have suggested that LRRK2 G2019S may be an α-synucleinopathy.5,11,12 Our patient, together with another reported case, confirms that the histopathology in patients with the G2019S mutation can be non-specific nigral degeneration without α-synuclein positive LB, and indicates that there is no correlation between the type of LRRK2 mutation and the underlying neuropathological changes.12 In addition, tau immunopositive neurofibrillary tangle pathology has been observed recently in a case of parkinsonism with the G2019S mutation.13 In light of this pleomorphic neuropathology associated with different LRRK2 mutations, some authors have hypothesised that the underlying pathogenic mechanism may be an upstream pathway of other proteins implicated in the pathogenesis of neurodegeneration.2
The G2019S mutation has also been observed in a control subject without a personal or family history of neurological or neurodegenerative disease who died at age 68 years. His neuropathological examination did not disclose any neurodegenerative abnormalities.11 The G2019S mutation was also found in a 89-year-old patient with neuropathologically confirmed Alzheimer’s disease.11 Both cases may reflect the incomplete penetrance of the LRRK2 mutations, which is probably influenced by age and other genetic and environmental factors.
Parkinsonism in the patient reported here showed a benign course for the first 10 years. The rapid worsening of the illness over the last 4 years, with major gait disturbances, could be explained by the development of ischaemic lesions involving the subcortical white matter and basal ganglia. Her initial benign parkinsonism may have been related to the final mild SN neuronal loss found at the pathological examination. Another explanation for this benign course is the absence of α-synuclein positive LB pathology. Patients with other genetic causes of PD syndrome, such as those with Parkin gene mutations, may also present with nigral degeneration without α-synuclein inclusions and frequently have less progressive parkinsonism compared with idiopathic PD patients.14,15 Therefore, it is reasonable to hypothesise that the absence of α-synuclein positive LB pathology may be a marker of a less aggressive pathogenic process with a slower rate of neuronal loss in the SN.
The presence of Marinesco bodies found in our patient has been described in neurons from other LRRK2 patients.2 Marinesco bodies are nuclear ubiquitin positive inclusions found in pigmented neurons of the SN nigra that increase in frequency with advancing age. Although no pathological associations have been clearly established, it has recently been suggested that these nuclear inclusions may not represent a benign phenomenon as they are associated with a significant decline in striatal dopaminergic markers.16 Furthermore, pigmented neurons of the SN containing LB more likely present Marinesco bodies than those pigmented neurons without LB, and proteasome dysfunction can lead to similar abnormalities in cultured cells.16 Although their pathological role is still unclear, Marinesco bodies may represent the accumulation or aggregation of ubiquitinated proteins induced by dysfunction of the ubiquitin–proteosome pathway and, consequently, it is possible that they may be involved directly in dopaminergic cell death or, in contrast, may represent an epiphenomenon in response to the risk of neuronal cell death.
Although LB disease is the most frequent autopsy finding in the cases reported in the literature, the present case confirms that clinical PD caused by G2019S mutations can be associated with non-specific nigral degeneration, without LB, α-synuclein or any other distinctive inclusion. More extensive series, autopsy and brain bank based, are necessary to clearly define the underlying neuropathology associated with each LRRK2 mutation
LEARNING POINTS
LRRK2 mutations are relatively common cause of Parkinson’s disease.
LRRK2 and sporadic Parkinson’s disease are clinically identical.
However, in the case described here an LRRK2 mutation was associated with non-specific nigral degeneration without typical Lewy body pathology of Parkinson’s disease.
Acknowledgments
This article has been adapted with permission from Gaig C, Marti MJ, Ezquerra M, Cardozo A, Rey MJ, Tolosa E. G2019S LRRK2 mutation causing Parkinson’s disease without Lewy bodies. J Neurol Neurosurg Psychiatry 2007;78:626–8.
Footnotes
Competing interests: none.
REFERENCES
- 1.Paisán-Ruiz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 2004; 44: 595–600 [DOI] [PubMed] [Google Scholar]
- 2.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004; 44: 601–7 [DOI] [PubMed] [Google Scholar]
- 3.Di Fonzo A, Tassorelli C, De Mari M, et al. Comprehensive analysis of the LRRK2 gene in sixty families with Parkinson’s disease. Eur J Hum Genet 2006; 14: 322–31 [DOI] [PubMed] [Google Scholar]
- 4.Nichols WC, Pankratz N, Hernandez D, et al. Genetic screening for a single common LRRK2 mutation in familial Parkinson’s disease. Lancet 2005; 365: 410–12 [DOI] [PubMed] [Google Scholar]
- 5.Gilks WP, Abou-Sleiman PM, Gandhi S, et al. A common LRRK2 mutation in idiopathic Parkinson’s disease. Lancet 2005; 365: 415–16 [DOI] [PubMed] [Google Scholar]
- 6.Gaig C, Ezquerra M, Martí MJ, et al. LRRK2 mutations in Spanish patients with Parkinson’s disease: frequency, clinical features and incomplete penetrance. Arch Neurol 2006; 63: 377–82 [DOI] [PubMed] [Google Scholar]
- 7.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55: 181–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funayama M, Hasegawa K, Ohta E, et al. An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Ann Neurol 2005; 57: 918–21 [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa K, Kowa H. Autosomal dominant familial Parkinson disease: older onset of age, and good response to levodopa therapy. Eur Neurol 1997; 38(Suppl 1): 39–43 [DOI] [PubMed] [Google Scholar]
- 10.Khan NL, Jain S, Lynch JM, et al. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson’s disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain 2005; 128: 2786–96 [DOI] [PubMed] [Google Scholar]
- 11.Ross OA, Toft M, Whittle AJ, et al. LRRK2 and Lewy body disease. Ann Neurol 2006; 59: 388–93 [DOI] [PubMed] [Google Scholar]
- 12.Giasson BI, Covy JP, Bonini NM, et al. Biochemical and pathological characterization of LRRK2. Ann Neurol 2006; 59: 315–22 [DOI] [PubMed] [Google Scholar]
- 13.Rajput A, Dickson DW, Robinson CA, et al. Lrrk2 G2019S, and tau neuropathology. Neurology 2006; 67: 1506–8 [DOI] [PubMed] [Google Scholar]
- 14.Mori H, Kondo T, Yokochi M, et al. Pathological and biochemical studies of juvenile parkinsonism linked to chromosome 6q. Neurology 1998; 51: 890–2 [DOI] [PubMed] [Google Scholar]
- 15.Khan NL, Graham E, Critchley P, et al. Parkin disease: a phenotypic study of a large case series. Brain 2003; 126: 1279–92 [DOI] [PubMed] [Google Scholar]
- 16.Beach TG, Walker DG, Sue LI, et al. Substantia nigra Marinesco bodies are associated with decreased striatal expression of dopaminergic markers. J Neuropathol Exp Neurol 2004; 63: 329–37 [DOI] [PubMed] [Google Scholar]