Abstract
The case is reported of an elite, male, white endurance runner (28 years of age), who is one of the best non-African runners in the world despite carrying the C34T mutation in the gene (AMPD1) that encodes the skeletal muscle specific isoform of AMP deaminase, an enzyme important in muscle metabolism. The frequency of the mutant allele in sedentary white people is 8–11%. Previous research has shown that this mutation, at least in homozygotes, can impair the exercise capacity of untrained people and their trainability. The maximum oxygen uptake (VO2MAX) of the study subject was exceptionally high (83.6 mlO2/kg/min), whereas his ammonia and lactate concentrations at high submaximal running speeds were lower than those of other world class runners who are not carriers of the mutation. The partial metabolic deficiency of the study subject is possibly compensated for by his exceptionally favourable anthropometric characteristics (body mass index 18.2 kg/m2).
BACKGROUND
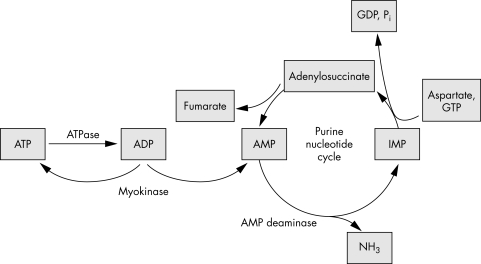
Adenosine monophosphate deaminase (AMPD) is an important regulator of muscle energy metabolism: by converting AMP into inosine monophosphate (IMP) with liberation of ammonia, this enzyme displaces the equilibrium of the myokinase reaction towards ATP production.1,2 Also, the AMPD reaction is the initial reaction of the purine nucleotide cycle (fig 1), which plays a central role in the salvage of adenine nucleotides and in determining energy charge.1 The skeletal muscle specific isoform of AMPD is encoded by the AMPD1 gene. A nonsense mutation (C34T) resulting in premature stopping of protein synthesis is the main cause of AMPD deficiency in white people.3 The frequency of the mutant allele in sedentary white people is 8–11%, and only 2% of the general white population is homozygous for this mutation (20% are heterozygous).1,4 The activity of muscle AMPD is greatly reduced even in heterozygous individuals, representing only 38–39% of its activity in healthy controls.5,6 Further, in some heterozygotes the activity of the enzyme can be as low as 16% of its normal activity.7 As a result, some degree of impairment in exercise capacity associated with the C34T mutation cannot be ruled out, even in heterozygotes. For instance, Norman et al7 reported that young people heterozygous for the C34T mutation have reduced capacities to deplete ATP pools and accumulate IMP during high intensity exercise—for example, higher (+18%) and lower (–32%) concentrations of ATP and IMP respectively—compared with normal homozygotes.
Figure 1. The purine nucleotide cycle.
A recent study by Rubio et al4 with 104 elite white endurance athletes (professional cyclists and runners) has shown that indices of endurance performance—for example, maximal oxygen uptake (V·O2MAX)—do not differ between athletes who are carriers (heterozygotes) and non-carriers of the C34T mutation. However, the frequency of the mutant allele was 50% lower (p<0.05) in the group of athletes (4.3%) compared with healthy sedentary controls (8.5%), and no athlete was homozygous for this mutation. Thus a certain degree of impairment in top level endurance performance due to the C34T mutation should not be rejected. In the aforementioned study,4 the physiological responses of endurance athletes could not be studied in depth because of the large population sample. In particular, ammonia concentrations were not determined during constant load exercise at high, submaximal intensities. Thus it would be interesting to study in more detail the specific physiological characteristics of the few elite endurance athlete carriers of this mutation, especially those who have attained the highest competition level, and to compare these data with those of the world’s best performers who are not carriers of the mutation
CASE PRESENTATION
We report the case of an elite white (Spanish) endurance (cross country and 5000–10 000 m races) runner (28 years of age). He is currently one of the best non-African runners in the world—that is, number one in Spain; in the top two in European cross country championships (12 km distance); in the top eight in the 5000 m at the World Championships; best time in 5000 and 10 000 m track races <13 min 20 s and <27 min 30 s respectively—despite being heterozygous for the C34T mutation in AMPD1. The C34T mutation was detected from venous blood samples using primers and polymerase chain reaction conditions previously indicated by Tsujino et al.8
INVESTIGATIONS
He performed a treadmill graded test until exhaustion to detect his maximal oxygen uptake (VO2MAX), and three treadmill running bouts (1.0% inclination) of six minutes duration at 17, 19, and 21 km/h respectively (on a separate day), to determine running economy—that is, mean VO2 for the last three minutes of each bout. Gas exchange data were collected continuously during exercise using an automated breath by breath system (Vmax 29C; Sensormedics, Yorba Linda, California, USA). Capillary blood samples (fingertips) were taken immediately on completion of each bout to determine lactate (YSI 1500; Yellow Springs Instruments, Yellow Springs, Ohio, USA) and ammonia (Menarini Diagnostics, Barcelona, Spain) concentrations. The results were compared with those of five of the best Spanish runners (mean age 27 years) and four world class elite East-African runners (23 years of age), who are not carriers of the C34T mutation. All are specialists in the same running events (cross country and 5000–10 000 m track events). All exercise tests were (a) preceded by a 24 hour period with no hard training and a total carbohydrate intake of 400 g and (b) performed under similar environmental conditions (20–24°C, 45–55% relative humidity) and at the same time of day (1200–1500). The subjects performed the exercise tests three hours after consuming a breakfast containing 100 g carbohydrate and no caffeinated drinks.
OUTCOME AND FOLLOW-UP
The height (162 cm), body mass (47.8 kg), and body mass index (18.2 kg/m2) of the subject were lower than in the other Spanish (173 cm; 61.8 kg; 20.7 kg/m2) or African (173 cm; 57.8 kg; 19.3 kg/m2) runners. His VO2MAX was exceptionally high (83.6 mlO2/kg/min), clearly above the mean values of the Spanish and African runners (75.8 and 74.5 mlO2/kg/min respectively). Overall, both lactate (0.9, 1.8, and 5.0 mmol/l) and, in particular, ammonia (52, 86, and 92 μmol/l) concentrations at 17, 19, and 21 km/h were lower in the runner with the mutation than in the Spanish (lactate: 1.4 (0.9) (0.9–2.9), 3.0 (1.0) (2.1–5.2), and 5.6 (1.1) (4.2–6.8) mmol/l; ammonia: 73(18) (55–85), 98 (24) (80–140), and 124 (14) (109–135) μmol/l) or African (lactate: 2.5 (0.5) (1.9–2.9), 3.4 (0.8) (2.5–4.0), and 4.3 (0.3) (3.9–4.5) mmol/l; ammonia: 97 (12) (81–106), 110 (25) (82–129), and 148 (11) (138–159) μmol/l) runners, who do not carry the mutation (all values are mean (SD) (range)). The VO2 cost of running was higher in the study subject at 17, 19, and 21 km/h (62.4, 70.4, and 79.3 mlO2/kg/min) than his Spanish counterparts (58.3 (2.8) (54.8–64.0), 67.1 (3.9) (62.3–72.5), and 72.5 (5.5) (63.5–81.0) mlO2/kg/min), and clearly higher than that of the Africans, especially at race speeds (21 km/h) (50.3 (6.3) (41.8–57.3), 59.0 (5.6) (51.5–65.6), and 65.2 (7.2) (52.3–72.7) mlO2/kg/min).
DISCUSSION
A continuous near-maximal effort (⩾90%VO2MAX) is required in 5000 and 10 000 m track events and cross country races during which glycogen and total adenine nucleotide pools are gradually depleted.4 During these very intense events, AMPD is likely to play an important metabolic role in working muscles, by converting AMP into IMP in the first reaction of the purine nucleotide cycle.1,4 The purine nucleotide cycle plays a central role in the salvage of adenine nucleotides and in determining energy charge (as it contributes, at least partly, to tricarboxylic acid cycle anaplerosis).1 However, partial deficiency of AMPD does not seem to have a negative impact on the competition performance of our study subject. Together with his very low body mass index, some physiological compensations (which have been reported in non-athletes who are carriers of the same mutation) may counterbalance the partial metabolic deficiency of this runner—for example, higher oxidative metabolism as a result of increased adenosine concentrations enhancing blood flow and increased ADP concentrations stimulating oxidative phosphorylation in working muscles respectively.7 (The VO2MAX of our subject who was partially deficient in AMPD was exceptionally high, reflecting a very high muscle oxidative capacity.) Also, although more research is necessary, the lower ammonia concentrations associated with AMPD deficiency may confer some performance benefit on endurance athletes, as, during prolonged strenuous exercise, elevated concentrations of blood ammonia result in increased cerebral uptake and accumulation of this metabolite, with subsequent occurrence of central fatigue—for example, through altered neurotransmitter metabolism and impaired voluntary activation of motor neurons.9
LEARNING POINTS
The C34T mutation of the AMPD1 gene may alter performance because it encodes an enzyme that plays an important metabolic role during strenuous exercise—that is, AMP deaminase
This case report illustrates that heterozygosity for this mutation does not necessarily impair top level endurance running performance
The lower ammonia concentrations associated with AMPD deficiency may confer some performance benefit on endurance athletes
Acknowledgments
This study was supported by a grant from Consejo Superior de Deportes (CSD, ref. #01/UPR/10/08) and Fondo de Investigaciones Sanitarias (FIS)
This article has been adapted with permission from Lucia A, Martin MA, Esteve-Lanao J, San Juan AF, Rubio JC, Oliván J, Arenas J. C34T mutation of the AMPD1 gene in an elite white runner. Br J Sports Med 2006;40:e7.
Footnotes
Competing interests: None.
REFERENCES
- 1.Rico-Sanz J, Rankinen T, Joanisse DR, et al. Associations between cardiorespiratory responses to exercise and the C34T AMPD1 gene polymorphism in the HERITAGE Family study. Physiol Genomics 2003; 14: 161–6 [DOI] [PubMed] [Google Scholar]
- 2.Lowenstein JM. Ammonia production in muscle and other tissues: the purine nucleotide cycle. Physiol Rev 1972; 52: 382–414 [DOI] [PubMed] [Google Scholar]
- 3.Morisaki T, Gross M, Morisaki H, et al. Molecular basis of AMP deaminase deficiency in skeletal muscle. Proc Natl Acad Sci USA 1992; 89: 6457–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubio JC, Martin MA, Rabadan M, et al. Frequency of the C34T mutation of the AMPD1 gene in World-class endurance athletes: does this mutation impair performance? J Appl Physiol 2005; 98: 2108–12 [DOI] [PubMed] [Google Scholar]
- 5.Norman B, Glenmark B, Jansson E. Muscle AMP deaminase deficiency in 2% of healthy population. Muscle Nerve 1995; 18: 239–41 [DOI] [PubMed] [Google Scholar]
- 6.Tarnopolsky MA, Parise G, Gibala MJ, et al. Myoadenylate deaminase deficiency does not affect muscle anaplerosis during exhaustive exercise in humans. J Physiol 2001; 533: 881–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norman B, Sabina RL, Jansson E. Regulation of skeletal muscle ATP catabolism by AMPD1 genotype during sprint exercise in asymptomatic subjects. J Appl Physiol 2001; 91: 258–64 [DOI] [PubMed] [Google Scholar]
- 8.Tsujino S, Shanske S, Carroll JE, et al. Double trouble: combined myophosphorylase and AMP deaminase deficiency in a child homozygous for nonsense mutations at both loci. Neuromuscul Disord 1995; 5: 263–6 [DOI] [PubMed] [Google Scholar]
- 9.Nybo L, Dalsgaard MK, Steensberg A, et al. Cerebral ammonia uptake and accumulation during prolonged exercise in humans. J Physiol 2005; 563: 285–90 [DOI] [PMC free article] [PubMed] [Google Scholar]