Abstract
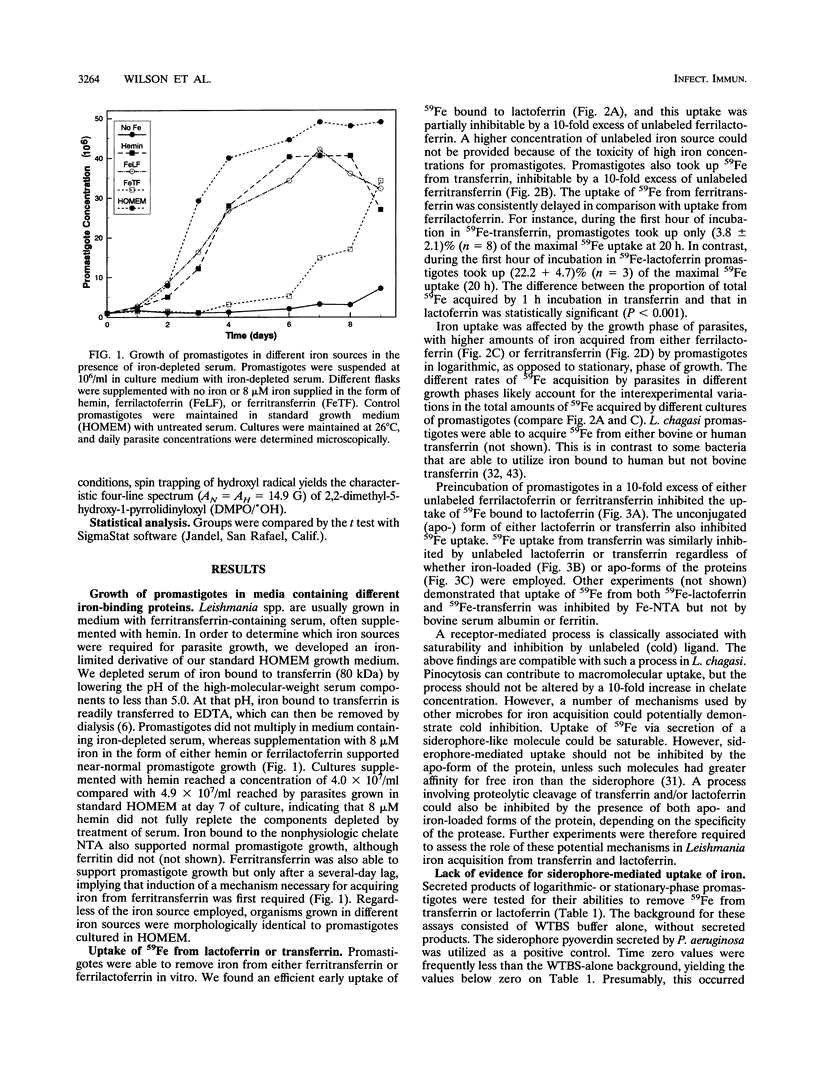
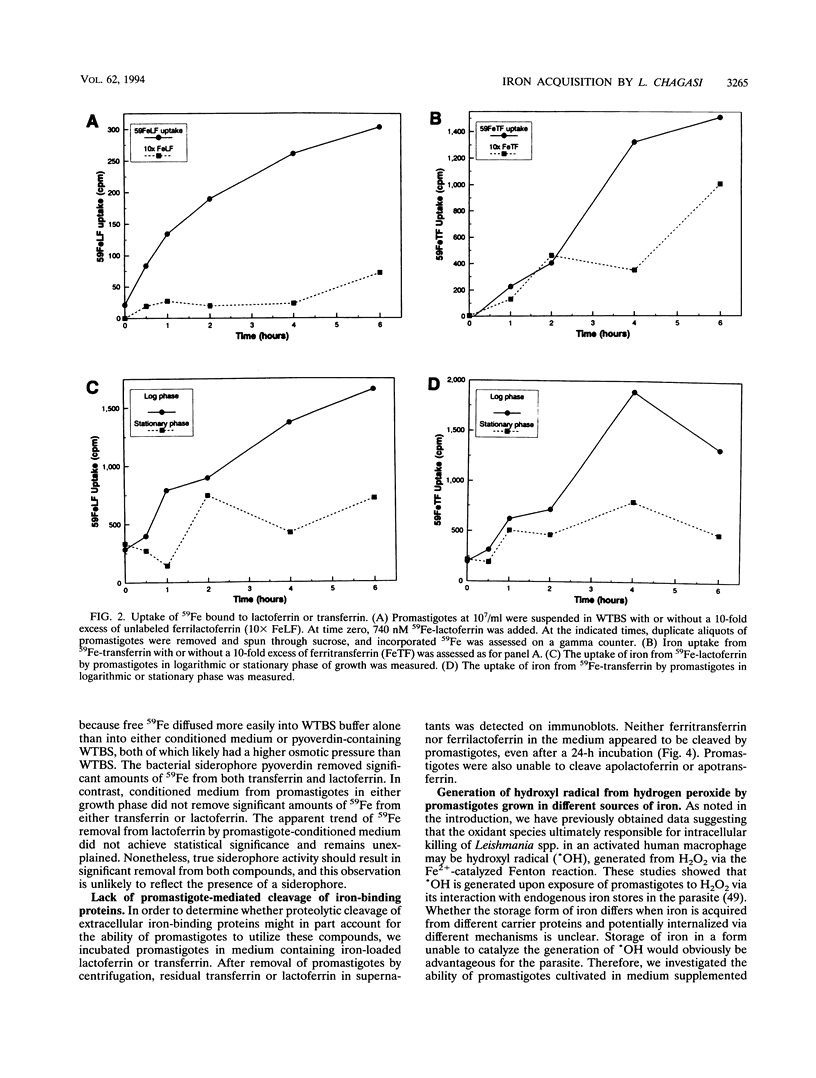
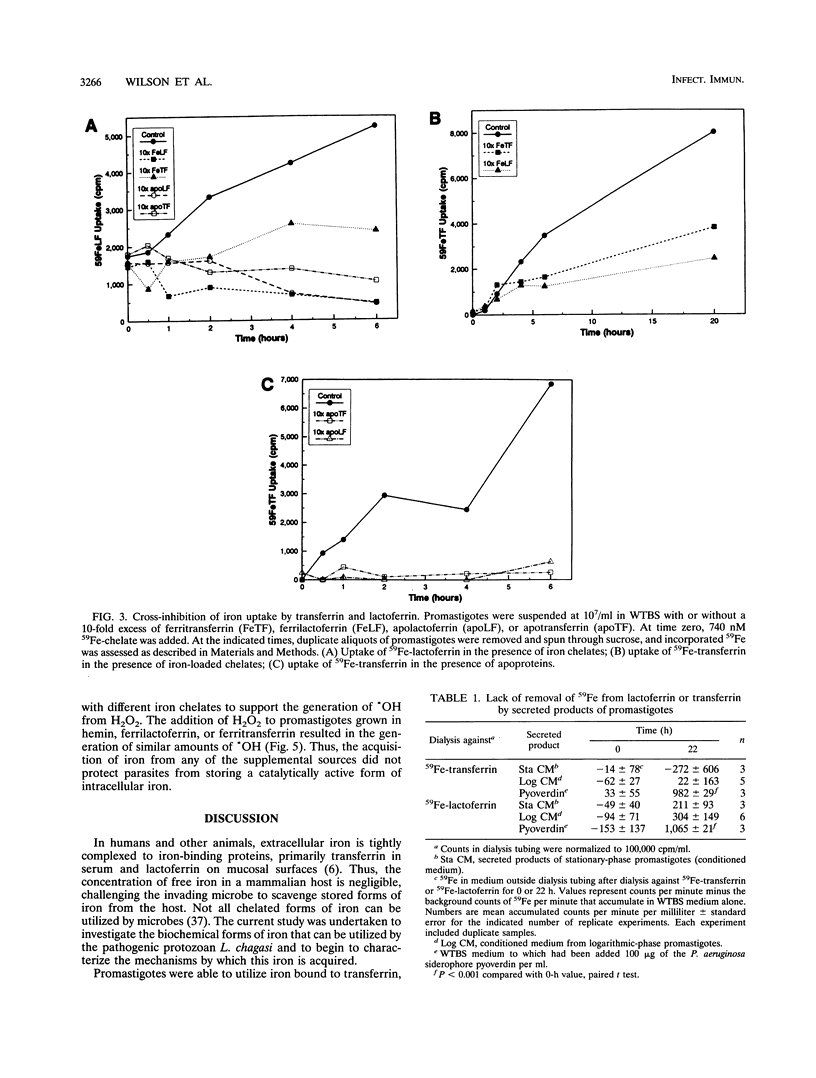
Leishmania chagasi, the cause of South American visceral leishmaniasis, requires iron for its growth. However, the extent to which different iron sources can be utilized by the parasite is not known. To address this question, we studied acquisition of iron from lactoferrin and transferrin by the extracellular promastigote form of L. chagasi during growth in vitro. A promastigote growth medium based on minimal essential medium supplemented with iron-depleted serum supported promastigote growth only after the addition of exogenous iron. The addition of 8 microM iron chelated to lactoferrin or hemin resulted in normal promastigote growth. Ferritransferrin also supported promastigote growth, but only after a considerable lag. Promastigotes grown in all three iron sources generated similar amounts of hydroxyl radical upon exposure to hydrogen peroxide, indicating that none of these protected parasites against generation of this toxic radical. Promastigotes were able to take up 59Fe chelated to either transferrin or lactoferrin, although uptake from 59Fe-lactoferrin occurred more rapidly. 59Fe uptake from either 59Fe-transferrin or 59Fe-lactoferrin was inhibited by a 10-fold excess of unlabeled ferrilactoferrin, ferritransferrin, apolactoferrin, apotransferrin, or iron nitrilotriacetate but not ferritin or bovine serum albumin. There was no evidence for a role for parasite-derived siderophores or proteolytic cleavage of ferritransferrin or ferrilactoferrin in the acquisition of iron by promastigotes. Thus, L. chagasi promastigotes can acquire iron from hemin, ferrilactoferrin, or ferritransferrin. This capacity to utilize several iron sources may contribute to the organism's ability to survive in the diverse environments it encounters in the insect and mammalian hosts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascencio F., Ljungh A., Wadström T. Lactoferrin binding properties of Vibrio cholerae. Microbios. 1992;70(283):103–117. [PubMed] [Google Scholar]
- Bartfeld N. S., Law J. H. Isolation and molecular cloning of transferrin from the tobacco hornworm, Manduca sexta. Sequence similarity to the vertebrate transferrins. J Biol Chem. 1990 Dec 15;265(35):21684–21691. [PubMed] [Google Scholar]
- Blanton K. J., Biswas G. D., Tsai J., Adams J., Dyer D. W., Davis S. M., Koch G. G., Sen P. K., Sparling P. F. Genetic evidence that Neisseria gonorrhoeae produces specific receptors for transferrin and lactoferrin. J Bacteriol. 1990 Sep;172(9):5225–5235. doi: 10.1128/jb.172.9.5225-5235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Hayek M. B., Doebbeling B. N., Fick R. B., Jr Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect Immun. 1993 Dec;61(12):5049–5055. doi: 10.1128/iai.61.12.5049-5055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Ward C. G., Rogers H. J. The critical role of iron in some clinical infections. Eur J Clin Microbiol Infect Dis. 1991 Aug;10(8):613–617. doi: 10.1007/BF01975810. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Chang K. P. Heme requirement and acquisition by extracellular and intracellular stages of Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1985 Sep;16(3):267–276. doi: 10.1016/0166-6851(85)90069-6. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985 Apr;48(1):130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J. H., Lee Y. S., Wong H. C. Effects of iron limitation on production of a siderophore, outer membrane proteins, and hemolysin and on hydrophobicity, cell adherence, and lethality for mice of Vibrio parahaemolyticus. Infect Immun. 1992 Jul;60(7):2952–2956. doi: 10.1128/iai.60.7.2952-2956.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Pfestorf M., Botzenhart K., Abdallah M. A. Impact of proteases on iron uptake of Pseudomonas aeruginosa pyoverdin from transferrin and lactoferrin. Infect Immun. 1988 Jan;56(1):291–293. doi: 10.1128/iai.56.1.291-293.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S., Cooper R. A., Evans R. W., Hider R. C., Williams P. H. Domain preference in iron removal from human transferrin by the bacterial siderophores aerobactin and enterochelin. Eur J Biochem. 1988 Dec 15;178(2):477–481. doi: 10.1111/j.1432-1033.1988.tb14473.x. [DOI] [PubMed] [Google Scholar]
- Galbraith R. A., McElrath M. J. Heme binding to Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1988 May;29(1):47–53. doi: 10.1016/0166-6851(88)90118-1. [DOI] [PubMed] [Google Scholar]
- Goldberg M. B., Boyko S. A., Calderwood S. B. Positive transcriptional regulation of an iron-regulated virulence gene in Vibrio cholerae. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1125–1129. doi: 10.1073/pnas.88.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar K., Henderson C. L., Cross G. A. Identification of the parasite transferrin receptor of Plasmodium falciparum-infected erythrocytes and its acylation via 1,2-diacyl-sn-glycerol. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8565–8569. doi: 10.1073/pnas.83.22.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Langford P. R., Towner K. J., Williams P. Evidence for in vivo expression of transferrin-binding proteins in Haemophilus influenzae type b. Infect Immun. 1992 Jul;60(7):2986–2991. doi: 10.1128/iai.60.7.2986-2991.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamroz R. C., Gasdaska J. R., Bradfield J. Y., Law J. H. Transferrin in a cockroach: molecular cloning, characterization, and suppression by juvenile hormone. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1320–1324. doi: 10.1073/pnas.90.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W., Varner L., Poch M. Acquisition of iron by Legionella pneumophila: role of iron reductase. Infect Immun. 1991 Jul;59(7):2376–2381. doi: 10.1128/iai.59.7.2376-2381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketola-Pirie C. A. Characterization of an insect ferritin subunit synthesized in a cell-free system. Biochem Cell Biol. 1990 Jul-Aug;68(7-8):1005–1011. doi: 10.1139/o90-148. [DOI] [PubMed] [Google Scholar]
- Kishore A. R., Erdei J., Naidu S. S., Falsen E., Forsgren A., Naidu A. S. Specific binding of lactoferrin to Aeromonas hydrophila. FEMS Microbiol Lett. 1991 Sep 15;67(1):115–119. doi: 10.1016/0378-1097(91)90454-i. [DOI] [PubMed] [Google Scholar]
- Lehker M. W., Arroyo R., Alderete J. F. The regulation by iron of the synthesis of adhesins and cytoadherence levels in the protozoan Trichomonas vaginalis. J Exp Med. 1991 Aug 1;174(2):311–318. doi: 10.1084/jem.174.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M. F., Villalta F. Trypanosoma cruzi receptors for human transferrin and their role. Mol Biochem Parasitol. 1990 Jan 15;38(2):245–252. doi: 10.1016/0166-6851(90)90027-j. [DOI] [PubMed] [Google Scholar]
- McGowan S. E., Henley S. A. Iron and ferritin contents and distribution in human alveolar macrophages. J Lab Clin Med. 1988 Jun;111(6):611–617. [PubMed] [Google Scholar]
- Menozzi F. D., Gantiez C., Locht C. Identification and purification of transferrin- and lactoferrin-binding proteins of Bordetella pertussis and Bordetella bronchiseptica. Infect Immun. 1991 Nov;59(11):3982–3988. doi: 10.1128/iai.59.11.3982-3988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J Exp Med. 1981 May 1;153(5):1302–1315. doi: 10.1084/jem.153.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu A. S., Miedzobrodzki J., Musser J. M., Rosdahl V. T., Hedström S. A., Forsgren A. Human lactoferrin binding in clinical isolates of Staphylococcus aureus. J Med Microbiol. 1991 Jun;34(6):323–328. doi: 10.1099/00222615-34-6-323. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Ogunnariwo J. A., Schryvers A. B. Iron acquisition in Pasteurella haemolytica: expression and identification of a bovine-specific transferrin receptor. Infect Immun. 1990 Jul;58(7):2091–2097. doi: 10.1128/iai.58.7.2091-2097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B. R., Verweij-van Vught A. M., MacLaren D. M. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol. 1992;18(3):217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Harcus J. L., Roberts D., Donowitz G. R. Differential survival of Leishmania donovani amastigotes in human monocytes. J Immunol. 1983 Oct;131(4):1994–1999. [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T. Mechanism of lethal effect of human serum upon Leishmania donovani. J Immunol. 1980 Nov;125(5):2195–2201. [PubMed] [Google Scholar]
- Pickett C. L., Auffenberg T., Pesci E. C., Sheen V. L., Jusuf S. S. Iron acquisition and hemolysin production by Campylobacter jejuni. Infect Immun. 1992 Sep;60(9):3872–3877. doi: 10.1128/iai.60.9.3872-3877.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta P. F., Turco S. J., McConville M. J., Lawyer P. G., Perkins P. V., Sacks D. L. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992 Jun 26;256(5065):1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- Pollack S., Schnelle V. Inability to detect transferrin receptors on P. falciparum parasitized red cells. Br J Haematol. 1988 Jan;68(1):125–129. doi: 10.1111/j.1365-2141.1988.tb04190.x. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Development of infective stage Leishmania promastigotes within phlebotomine sand flies. Am J Trop Med Hyg. 1985 May;34(3):456–459. doi: 10.4269/ajtmh.1985.34.456. [DOI] [PubMed] [Google Scholar]
- Schell D., Evers R., Preis D., Ziegelbauer K., Kiefer H., Lottspeich F., Cornelissen A. W., Overath P. A transferrin-binding protein of Trypanosoma brucei is encoded by one of the genes in the variant surface glycoprotein gene expression site. EMBO J. 1991 May;10(5):1061–1066. doi: 10.1002/j.1460-2075.1991.tb08045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B., Gonzalez G. C. Receptors for transferrin in pathogenic bacteria are specific for the host's protein. Can J Microbiol. 1990 Feb;36(2):145–147. doi: 10.1139/m90-026. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B. Identification of the transferrin- and lactoferrin-binding proteins in Haemophilus influenzae. J Med Microbiol. 1989 Jun;29(2):121–130. doi: 10.1099/00222615-29-2-121. [DOI] [PubMed] [Google Scholar]
- Soares M. J., Souto-Padrón T., De Souza W. Identification of a large pre-lysosomal compartment in the pathogenic protozoon Trypanosoma cruzi. J Cell Sci. 1992 May;102(Pt 1):157–167. doi: 10.1242/jcs.102.1.157. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Voyiatzaki C. S., Soteriadou K. P. Evidence of transferrin binding sites on the surface of Leishmania promastigotes. J Biol Chem. 1990 Dec 25;265(36):22380–22385. [PubMed] [Google Scholar]
- Voyiatzaki C. S., Soteriadou K. P. Identification and isolation of the Leishmania transferrin receptor. J Biol Chem. 1992 May 5;267(13):9112–9117. [PubMed] [Google Scholar]
- Yu R. H., Gray-Owen S. D., Ogunnariwo J., Schryvers A. B. Interaction of ruminant transferrins with transferrin receptors in bovine isolates of Pasteurella haemolytica and Haemophilus somnus. Infect Immun. 1992 Jul;60(7):2992–2994. doi: 10.1128/iai.60.7.2992-2994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarley J. H., Britigan B. E., Wilson M. E. Hydrogen peroxide-mediated toxicity for Leishmania donovani chagasi promastigotes. Role of hydroxyl radical and protection by heat shock. J Clin Invest. 1991 Nov;88(5):1511–1521. doi: 10.1172/JCI115461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva R., Sacks D. L. Metacyclogenesis is a major determinant of Leishmania promastigote virulence and attenuation. Infect Immun. 1987 Nov;55(11):2802–2806. doi: 10.1128/iai.55.11.2802-2806.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



