Graphical abstract
Anti-inflammatory activity of a dwarf elder leaf extract (Sambucus ebulus L.) was assessed by activity guided fractionation using inhibition of TNFα induced expression of VCAM-1 on the surface of HUVECs as monitoring tool. The active principal was identified as ursolic acid (IC50 6.25 μM).
Keywords: Sambucus ebulus L., Adoxacea, Ursolic acid, VCAM-1, ICAM-1, HUVEC, TNF-α, Anti-inflammatory activity
Abstract
Aim of the study
The performed investigations aimed on the identification of the anti-inflammatory principal of extracts of leaves of Sambucus ebulus L. (dwarf elder) in order to rationalize the traditional use of this plant for the treatment of chronically inflammatory diseases.
Materials and methods
Dwarf elder leaf extract was subjected to activity guided fractionation using inhibition of TNFα induced expression of vascular cell adhesion molecule 1 (VCAM-1) on the surface of human umbilical vein endothelial cells (HUVECs) as monitoring tool (positive control: parthenolide 10 μM, VCAM-1 expression (% of control): 5.35 ± 0.38%).
Results
Bio-guided isolation resulted in identification of ursolic acid as anti-inflammatory principal. Besides its inhibitory effects against TNFα induced expression of VCAM-1 (IC50 6.25 μM), ursolic acid inhibits also TNFα induced expression of ICAM-1 (IC50 value between 3.13 and 6.25 μM) (positive control: parthenolide 10 μM, ICAM-1 expression (% of control): 38.89 ± 16.6%). Toxic effects of ursolic acid on HUVECs can be drastically reduced using an enriched extract instead of the pure compound.
Conclusions
Our findings suggest an additional mechanism of the anti-inflammatory activity of ursolic acid by demonstrating its ability to inhibit TNFα-stimulated expression of VCAM-1 and ICAM-1 and support the traditional use of extracts and preparations of Sambucus ebulus L., rich in ursolic acid, for the treatment of chronically inflammatory processes.
1. Introduction
Earliest written descriptions of the therapeutic use of dwarf elder (Sambucus ebulus L.; Adoxacea) extracts in humans can be found in Pliny the Elder's “Naturalis Historia” (23–79 A.D.) and in “De Materia Medica” of Dioscorides (40–90 A.D.). In traditional medicine, extracts from the root and leaves of Sambucus ebulus L. are frequently used for the treatment of inflammatory diseases such as inflammatory joint diseases, rheumatic pain and sore throat (Hiermann, 2007). Extracts of some aerial plant parts exhibited distinct effects in the carrageenan induced rat paw edema assay (Ebrahimzadeh et al., 2006). Leave extracts showed also an impact on the concentration of cytokines (interleukin-1α, interleukin-1β, TNFα) when mixed with whole blood of healthy volunteers (Yesilada et al., 1997). Despite the traditional use of the leaves of Sambucus ebulus L. especially in Mediterranean countries, up to now only one attempt was performed to identify the active principle (Yesilada, 1997). An extract of the aerial plant parts was subjected to activity guided isolation using different animal models (carrageenan or serotonin induced mice paw edema assays and others) ending up with identification of chlorogenic acid as active principle. Unfortunately, the whole work was published without any experimental results concerning the pharmacological part. This rather unclear situation together with the fact that we recently were able to isolate six new iridoid glycosides from the leaves of Sambucus ebulus L. (Pieri et al., 2009) with unknown pharmacological properties prompted us to perform an activity-guided isolation of an ethanolic extract of the leaves of Sambucus ebulus L. in order to identify the anti-inflammatory principal. Since there are several pathways involved in inflammatory processes, e.g. arachidonic acid pathway via COX1/2 and LOX subtypes, NFκB pathway; PPARs, LXR and many others, we had to decide which model would support the current state of investigations. With respect to the work of Yesilada et al. (1997) we decided to establish a very general model of inhibition of the TNFα induced expression of vascular cell adhesion molecule 1 (VCAM-1) on the surface of human umbilical vein endothelial cells (HUVECs). Increased expression of VCAM-1 is associated with a variety of chronic inflammatory conditions, making its expression and function a target for therapeutic intervention (Besemer et al., 2005). Besides the feasibility of high throughput screening this assay allows to monitor several targets affected by TNFα, e.g. NFκB activation are monitored at once (Nakanishi and Toi, 2005). The disadvantage of this model is the fact that you need another assay for controlling cell viability, since a reduced number of cells leads also to a reduction of detectable VCAM-1.
2. Materials and methods
2.1. General
All reagents used were of purissimum or analytical grade and were purchased from Sigma–Aldrich (Vienna, Austria) unless specified otherwise. HPLC solvents were of gradient grade and purchased from Sigma–Aldrich as well. Water was produced by reverse osmosis followed by distillation. Pure grade solvents were distilled prior to use. Ursolic acid purchased from Sigma was of 98.5% purity.
2.2. Cell isolation and culture
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords (kindly donated by the Gynaecology and Obstetrics Department, Innsbruck Medical University) by enzymatic detachment using collagenase, as previously described (Bernhard et al., 2003). Cells were routinely passaged in 0.2% gelatine-coated (Sigma, Steinheim, Germany) polysterene culture flasks (Becton Dickinson, Meylan Cedex, France) in Endothelial Cell Basal Medium (CC-3121, BioWhittaker, Inc., Walkersville, MD, USA) supplemented with EGM SingleQuots Supplements and growth factors (CC-4133, BioWhittaker, Inc.) in a humidified atmosphere containing 5% CO2. Fetal bovine serum (FBS; 2%) was routinely used for cell culturing and experiments. The isolation and analysis of human umbilical cord ECs were approved by the Ethics Committee of the Innsbruck Medical University.
2.3. Quantification of endothelial surface vascular cell adhesion molecule 1 (VCAM-1) expression
To analyze and quantify TNFα-induced VCAM-1 expression on the surface of HUVECs, 10,000 HUVECs were seeded into each well of a 96-well plate and allowed to adhere overnight. Following replacement of medium with 200 μL of fresh medium, different dilutions of plant extracts or solvents (as controls) were added to the wells. After an incubation period of 30 min, human recombinant TNFα (Peprotech (Eubio), 300-01A) was added to the wells at a final concentration of 100 ng/mL. After incubation for 15 h, supernatants were discarded, cells were washed five times in ice cold PBS, and the 96-well plates were put on ice. Following blocking of non-specific bindings sites with 1% BSA in PBS for 60 min, mouse anti-human CD106/VCAM-1 antibody (Neomarkers, MS-1101-P, final concentration 0.2 μg/mL in PBS, 1% BSA) was added to the wells or isotype control (Mouse IgG1, Dako, X0931, final concentration 0.2 μg/mL in PBS, 1% BSA) and incubated for 30 min on ice on a horizontal shaker. Following washing of cells five times in ice cold PBS, cells were incubated with HRP labelled secondary antibodies (goat anti-mouse HRP, Dako, P0447, final concentration 1 μg/mL in PBS, 1% BSA) for 30 min on ice. After another washing step (five times with ice cold PBS), 0.32 mg/mL of ABTS (Roche) in ABTS buffer (512 mg NaBO3-trihydrate, 8.799 g citric acid 1-hydrate, and Di-NaHPO4-dihydrate 11.214 g/l (pH 4.4)) was added to the wells and allowed to develop for 45 min in the dark. OD was determined at 405 nm. At least three dilutions per extract/fraction were tested in four parallels, and each experiment was repeated three to five times. Positive control: parthenolide (Sigma–Aldrich) 10 μM, VCAM-1 expression (% of control): 5.35 ± 0.38%. All data were verified by FACS-based quantification of VCAM-1 (data not shown).
2.4. Quantification of endothelial surface inter cellular adhesion molecule 1 (ICAM-1) expression
The protocol for the quantification of surface ICAM-1 on endothelial cells was identical to the protocol for VCAM-1 detection (see above). Antibodies used were: anti-mouse anti-human CD54/ICAM-1 antibody (Neomarkers, MS-1094-S, 1:15 in PBS, 1% BSA), isotype control (Mouse IgG1, Dako, X0931, final concentration 0.2 μg/mL in PBS, 1% BSA), HRP-labelled secondary antibodies (Goat anti-Mouse HRP, Dako, P0447, final concentration 1 μg/mL in PBS, 1% BSA). Positive control: parthenolide (Sigma–Aldrich) 10 μM, ICAM-1 expression (% of control): 38.89 ± 16.6%.
2.5. Analysis of the number of viable cells
The number of viable cells in the cultures was determined by the XTT assay (Biomol, Hamburg, Germany) according to the manufacturer's instructions.
2.6. Plant material
Leaves of Sambucus ebulus L. were collected in Gaaden (Carinthia/Austria) from an uncultivated site in September 2003. A herbarium specimen (CS-09200301) is stored at the Institute of Pharmacy/Pharmacognosy, University of Innsbruck. The fresh leaves (830 g) were air dried at room temperature in a shaded room for 14 days. The presented phytochemical work was carried out from October 2003 to September 2005.
2.7. Chromatography and NMR analyses
High-speed counter-current chromatography (= HSCCC): P.C. Inc. (Potomac; MD, USA; model: HSCCC multilayer (triple) coil, ser. 690) HSCCC instrument with Gilson 302/803 C pump system model 302 (Villiers-la-Bel, France). LC for quantification: LC-parameter: HP 1050 system (Agilent, Waldbronn, Germany) equipped with auto sampler, DAD and column thermostat; stationary phase: Phenomenex Luna 3μ C8 100A (150 mm × 3.0 mm) with Synergy Polar RP 80A guard column; mobile phase: solvent A: H2O with 0.02 trifluor acetic acid (v/v); solvent B: acetonitrile; DAD: 205 nm, temp.: 35.0 °C; injection volume: 5 μL; flow: 0.2 mL/min; composition during run: start: 60% A; 10 min: 30% A; 40 min: 2% A; 55 min: stop; post time: 15 min. LC–MS: LC-parameter: HP 1100 system (Agilent, Waldbronn, Germany) equipped with auto sampler, DAD and column thermostat; stationary phase: Phenomenex Synergy Polar RP 80A (150 mm × 4.6 mm) with identical guard column; mobile phase: solvent A: H2O with 0.9% formic acid, 0.1% acetic acid (v/v); solvent B: acetonitrile + methanol (v/v, 1 + 1); DAD: 250 nm, temp.: 40 °C; injection volume: 10 μL; flow: 1.0 mL/min; composition during run: start: 50% A; 20 min: 42% A; 21 min: 28% A; 40 min: 4% A; 45 min: stop; post time: 10 min. MS-parameters: Esquire 3000plus (Bruker Daltonics, Bremen, Germany); split: 1:5; ESI, alternating mode; spray voltage: −4.5 kV, 350 °C; dry gas: 10.00 L/min; nebulizer 40 psi; full scan mode: m/z 100–1500. TLC: silica gel 60 F254 plates (VWR Darmstadt, Germany) mobile phase: toluene + ethyl acetate (1 + 1; v/v), chemical derivatization with vanillin-sulfuric acid reagent. NMR: 1D- and 2D-experiments were measured on a Bruker DRX 300 (Bruker Biospin Rheinstetten, Germany) operating at 300.13 MHz (1H) and 75.47 MHz (13C) at 300 K (chemical shifts δ in ppm, coupling constants J in Hz); NMR solvent: DMSO-d6 with 0.03% TMS (Eurisotop Gif-Sur-Yvette, France), which was used as internal standard. Melting point (m.p.): Kofler hot-stage microscope; uncorr. Optical rotation: Perkin-Elmer 341 polarimeter (Wellesley, MA, USA) at 25 °C.
2.8. Extraction and separation
Extraction was carried out with 570 g of the milled air-dried leaves, which were macerated with 2.5 L EtOH96% for 24 h at room temperature. The mixture was sonicated for 10 min and filtered. The obtained solution was evaporated to dryness using a rotavapor at 25 °C. This procedure was repeated eight times with fresh solvent each time and collectively yielded 116.25 g crude extract. The initial separation was performed by means of liquid–liquid extraction; 95.23 g of the crude extract were suspended in 1.0 L water and transferred into the separatory funnel. The suspension was extracted six times with 500 mL of solvents of different polarity starting with petroleum ether, diethyl ether, ethyl acetate and finally water saturated n-butanol. The obtained fractions were evaporated to dryness yielding 18.94 g petroleum ether, 20.45 g diethyl ether, 7.69 g ethyl acetate, 18.24 g n-butanol and 35.10 g water fraction (highly hygroscopic). Since the activity was concentrated in the obtained diethyl ether fraction, 19.0 g of the fraction were subjected to silica gel column chromatography using 230 g Merck silica gel 60 (0.040–0.063 mm, 230–400 mesh) as stationary phase and a gradient of petroleum ether and increasing amounts of ethyl acetate as mobile phase. The eluate was monitored by TLC and combined to eight fractions (SE-D 1 to SE-D 8). The activity was located in fractions SED 4, 5 and 6. Due to the higher amount and purity of fraction SE-D 4 (2.55 g) compared to the total amount of SE-D 5 and 6 (1.50 g), the further separation steps were carried out with fraction SE-D 4. For further enrichment, SE-D 4 was divided in a dichloromethane (= DCM)-soluble and a DCM-insoluble part. For this purpose, 2.20 g of SE-D 4 were suspended in 10.0 mL DCM and sonicated for 5 min. The mixture was filtered over a sintered plate funnel (porosity nr. 4). The same procedure was repeated four times yielding two fractions: SE-Ak-f-DCM-soluble (0.48 g) and SE-Ak-f-DCM-insoluble (1.71 g). The further separation of the active fraction SE-Ak-f-DCM-insoluble was performed by means of liquid–liquid extraction using 1.64 g of SE-Ak-f-DCM-insoluble and 900 mL of a solvent mixture of petroleum ether, ethyl acetate, acetonitrile and tertiary butyl-methyl ether (10 + 1 + 5 + 2, all v/v). The evaporated upper layer yielded 1.10 g, the lower layer afforded yielded 0.54 g (SE-Ak-g). The residue of the lower layer was further purified by means of HSCCC using a solvent mixture of petroleum ether, ethyl acetate, acetonitrile and tertiary butyl-methyl ether (10 + 1 + 5 + 2, all v/v) using the upper layer as mobile phase. The sample (458.12 mg of SE-Ak-g) was suspended in 10.0 mL of the lower phase and 5.0 mL of the upper phase and filtered before injection yielding an insoluble part of 129.80 mg. The used coil had a volume of 325 mL and was used in the tail-to-head-modus with a rotation of 800 rpm. The used flow rate was 1.00 mL/min, fractions were collected in portions of 5 mL. Although the separation technique enabled a good separation of the compounds, which were combined to three fractions (SE-Ak-h 1–3), the activity remained in the insoluble part (SE-Ak-h RS). Since the residue (SE-Ak-h RS) formed pale yellow crystals, the insoluble part was subjected to crystallization with a mixture of acetonitrile and tetrahydrofuran (v/v; 1 + 1). The first crystallization afforded 67.7 mg of off-white platelets, which were crystallized a second time from the same solvent mixture yielding 47.3 mg of white platelets.
2.9. Structure elucidation of the active principle
LC–MS analysis of the crystallized residue afforded in positive mode m/z of 439.3; 457.3 and 479.4 corresponding to the pseudo-molecular ions of [M−H2O]+, [M+1]+ and [M+Na]+ as well as a m/z of 455.2 in negative mode corresponding to [M−1]− suggesting a molecular weight of 456 g/mol. The 1D and 2D NMR-analysis of 20.0 mg of the obtained compound enabled the identification of the isolated compound as ursolic acid (Tundis et al., 2002) with 85% purity.
2.10. Determination of ursolic acid concentration in the diethyl ether fraction
Quantification of the ursolic acid concentration was carried out by a HPLC-DAD method using the method of external standard with a calibration curve of y = 6446x + 98.509 (R2 = 0.9999). For this purpose, the commercial ursolic acid was dissolved in three concentrations (5 mM, 0.5 mM and 0.25 mM) in DMSO and analyzed by HPLC in triplicate. Determination of the ursolic acid concentration in DMSO solution in the obtained diethyl ether fraction (10 mg/mL) afforded a content of 14.79 ± 0.03% (w/w).
3. Results
3.1. Activity-guided isolation
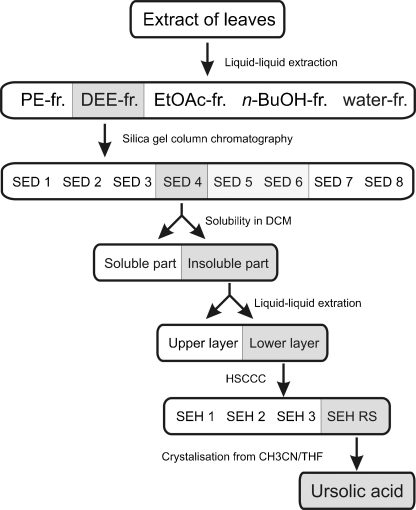
Results of the activity-guided fractionation are summarized in Fig. 1. Activity was evaluated monitoring the reduction of TNFα induced VCAM-1 expression of HUVECs. A crude ethanol leave extract which reduced VCAM-1 expression by 74.5% (c = 33 μg/mL) compared to control was used as starting material.
Fig. 1.
Activity guided isolation schema. Extract of the leaves of Sambucus ebulus L. was subjected to activity-guided fractionation monitoring the reduction of TNFα induced VCAM-1 expression of HUVECs. PE = petroleum ether, DEE = diethyl ether, EtOAc = ethyl acetate.
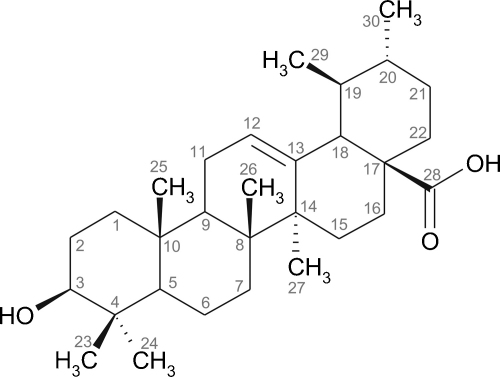
First, this extract was divided in five fractions by liquid–liquid extraction. Activity of the petroleum ether fraction was comparable to that of the starting ethanol extract, whereas the diethyl ether fraction caused an even more pronounced reduction of VCAM-1 expression (90.7%). The more polar fractions had little effects or were almost inactive (ethyl acetate: 39.4%, n-butanol: 15.2%, water: 13.9%). Silica gel column chromatography of the diethyl ether fraction yielded eight fractions. Fraction 4 (SED4), which showed potent activity, was separated into two fractions according to their solubility in DCM. The active DCM insoluble part was subjected to liquid/liquid partitioning followed by HSCCC. Of the resulting four fractions fraction 4 (SHE RS) turned out to contain the active principle. Final crystallization from CH3CN/THF yielded ursolic acid as major constituent. Its structure (Fig. 2) was confirmed by mass spectrometry and 1 and 2D NMR spectroscopy and is reported form this source the first time.
Fig. 2.
Chemical structure of ursolic acid.
3.2. Biological characteristics of the isolate and comparison with commercially available ursolic acid
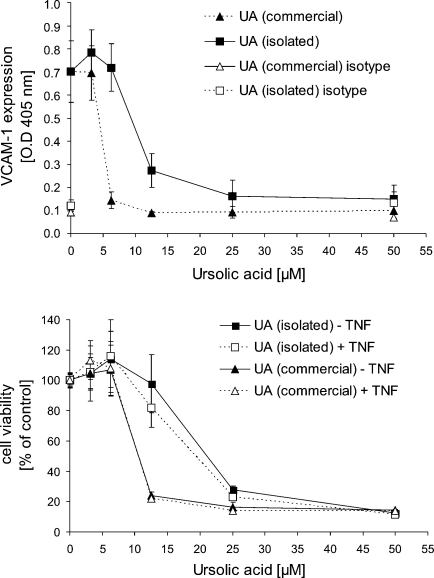
Ursolic acid isolated from the leaves of Sambucus ebulus L. (purity 85%) reduced TNFα induced VCAM-1 expression of HUVECs in a dose dependent manner with an IC50 value 12.5 μM. In comparison commercial ursolic acid (purity >98.5%) showed an IC50 value of 6.25 μM (Fig. 2; positive control: parthenolide 10 μM, VCAM-1% of control: 5.35 ± 0.38%). Besides inhibition of VCAM-1 expression purified and commercial ursolic acid significantly reduced viability of both endothelial and smooth muscle cells (SMCs). IC50 values of purified and commercial ursolic acid determined by the XTT assay (Fig. 3) were 22 μM and 11.5 μM, respectively. Viability data for SMCs were almost identical with those for ECs and are therefore not shown. Reason for the differences between ursolic acid we isolated from plant material and the commercially available compound may be the different grade of purity (85% compared to >98.5%). These findings prompted us to use commercial ursolic acid for all subsequent experiments.
Fig. 3.
Comparison of the activity of isolate and commercially available ursolic acid (UA). The upper diagram shows a comparison of the isolated vs. the commercial product on the basis of inhibition of VCAM-1 expression. To confirm specificity an isotype control was included in the experiments. Mean values of a representative experiment performed in quadruplicate ± S.D. are shown. Positive control: parthenolide 10 μM, VCAM-1 expression OD 405 nm: 0.114 ± 0.008. The lower diagram shows an analysis of cytotoxicity/cell viability by the XTT assay. Data represent mean values of a representative experiment performed in triplicate ± S.D. Cells were exposed to the compounds for 24 h (compounds were added 30 min prior to TNFα addition).
3.3. Ursolic acid inhibits TNFα-mediated ICAM-1 surface expression
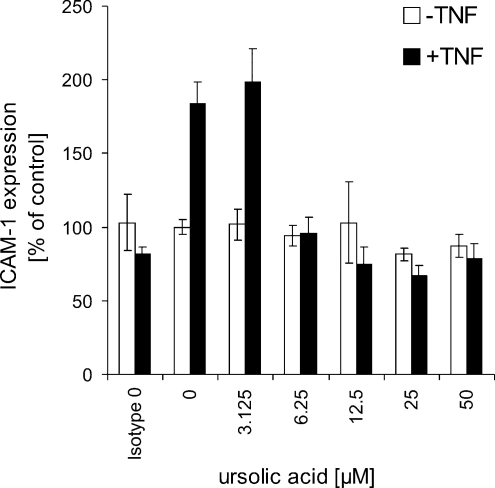
Since exposure of HUVECs to TNFα is known to cause not only expression of VCAM-1 but also expression of other adhesion molecules, we tested whether ursolic acid is antagonizing also ICAM-1 expression. Fig. 4 shows the outcomes: ursolic acid inhibits TNFα stimulated surface expression of ICAM-1 on endothelial cells in a dose dependent manner (IC50 value between 3.13 and 6.25 μM; positive control: parthenolide 10 μM, ICAM-1 expression (% of control): 38.89 ± 16.6%). In the absence of TNFα ursolic acid does not has any effect on ICAM-1 expression.
Fig. 4.
Ursolic acid inhibits TNFα-mediated ICAM-1 expression of endothelial cells. To confirm specificity an isotype control was included in the experiments. Mean values of a representative experiment performed in quadruplicate ± S.D. are shown. Positive control: parthenolide 10 μM, ICAM-1 expression (% of control): 38.89 ± 16.6%.
4. Discussion
Extracts of roots and leaves of Sambucus ebulus L. have been used in traditional medicine to treat health problems associated with inflammation such as inflammatory joint diseases, rheumatic pain and sore throat. Several studies have confirmed the anti-inflammatory potential of dwarf elder extracts, but the nature of the active principle remained unsolved. In the course of this project an activity guided isolation approach resulted in identification of ursolic acid as active principal for the inhibitory effect of dwarf elder leave extracts against TNFα induced HUVEC surface expression of VCAM-1. Interestingly, none of the recently identified iridoid derivatives (Pieri et al., 2009) was involved in the observed activity.
Ursolic acid is a widespread pentacyclic tripernoid and has previously been isolated from a number of different plants species, e.g. rosemary and basil. Recent reports have ascribed to ursolic acid chemo-preventive properties (Lu et al., 2007), cell death-inducing (Achiwa et al., 2005), anti-mutagenic (Ramos et al., 2008) and anti-viral activities (Serra et al., 1994), direct anti-oxidative properties (Ramachandran and Prasad, 2008), anti-inflammatory as well as pro-inflammatory properties (Ikeda et al., 2008), pro-angiogenic and antiangiogenic properties (Sohn et al., 1995; Cardenas et al., 2004), ability to induce differentiation (Lee et al., 1994), and anti-invasive activities (Cha et al., 1996). Findings that might be of relevance for an application of ursolic acid in cardiovascular medicine include vasorelaxant activity (Aguirre-Crespo et al., 2006) and an up-regulation of eNOS (Steinkamp-Fenske et al., 2007), as well as anti-oxidant activity, e.g. inhibition of lipid peroxidation (Balanehru and Nagarajan, 1991). In an insulin-resistant rat model of genetic hypertension, ursolic acid was further shown to have antihypertensive, heart rate-lowering, anti-hyperlipidemic, and anti-hyperglycemic effects (Somova et al., 2003).
Despite a few reports on pro-inflammatory effects, which may be based on specific cellular and tissue environments, ursolic acid is generally considered to act as an anti-inflammatory agent, e.g. by inhibition of COX-2 transcription (Subbaramaiah et al., 2000), release of macrophage migration inhibitory factor (Ikeda et al., 2005), inhibition of IL-8 secretion (Thuong et al., 2005), and downregulation of enzymes required for permeation of lymphocytes/monocytes through basal lamina such as MMP-9 and elastase (Cha et al., 1996). In general, a number of effects of ursolic acid on cells in culture were ascribed to an interaction with cellular signalling at or around NFκB activation and/or activity. However – to our knowledge – up to now only one report suggested a precise site of interaction of ursolic acid with NFκB, namely inhibition of IκBα-kinase and phosphorylation of p65 (Shishodia et al., 2003).
Our findings indicate that ursolic acid is able to inhibit TNFα-stimulated expression of vascular cell adhesion molecules as well as the expression of inter cellular adhesion molecules. While reduction of VCAM-1 corroborates the use of dwarf elder extracts for the therapy of chronically inflammatory processes, reduction of the TNFα-stimulated expression of ICAM-1 might at least partially explain the (traditional) use of different Sambucus species, e.g. flowers of S. nigra L. containing 0.72% ursolic acid according to Richter and Wilhun (1977) for the treatment of common cold. ICAM-1 serves as a crucial target-docking molecule on epithelial cells for 90% of human rhinovirus (HRV) serotypes, which are responsible for up to 65% of common colds (Whiteman and Spiteri, 2008).
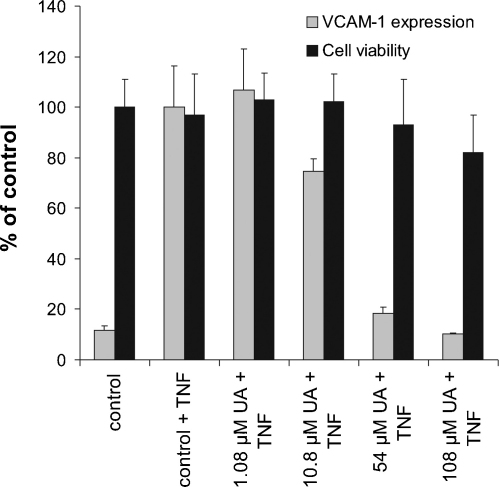
In agreement with literature data, our in vitro analyses showed that ursolic acid has a profound cell death-inducing activity. Interestingly, as constituent of an extract the toxicity of ursolic acid seems to be diminished. This was quite evident when comparing the dwarf elder diethyl ether fraction (DEE-fr. containing 14.79 ± 0.03% (w/w) ursolic acid) with the pure compound ursolic acid: despite its slightly lower VCAM-1 expression-repressing activity (Fig. 5; IC50 of 20 μM compared to 5–10 μM ursolic acid) it is significantly less toxic as suggested by the content of ursolic acid in the extract demonstrated a clearly more pronounced reduction of toxicity (IC50 > 108 μM compared to 10–20 μM ursolic acid).
Fig. 5.
Effect of diethyl ether fraction adjusted to the corresponding ursolic acid concentration on VCAM-1 expression (light bars) and cell viability (dark bars). Shown are representative experiments performed in quadruplicates ± S.D. Positive control: parthenolide (10 μM); VCAM-1 expression (% of control): 5.35 ± 0.38%.
One reason for the observed alleviation of side effects might be related to an induction of phase II detoxification enzymes. Since also chlorophyll, which is present in a high amount in the diethyl ether fraction, is able to induce phase II enzymes like heme oxygenase-1 (= HO-1) and quinone oxidoreductase-1 (= NQO1) (Zhang et al., 2008) it can be assumed that the observed effect is a result of an interaction between ursolic acid and present chlorophyll derivatives. The verification of this hypothesis is part of an ongoing research project.
5. Conclusions
Results from this study clearly showed that extracts and fractions of the leaves of Sambucus ebuluss L. are able to inhibit TNFα-stimulated expression of VCAM-1 and ICAM-1 in HUVECs. The responsible principal for this effect was identified as ursolic acid. Our findings support the traditional use of extracts and preparations of Sambucus ebulus L., rich in ursolic acid, for the treatment of chronically inflammatory processes.
Sources of funding
This project was supported by the Austrian National Bank (Project 12697 to DB), the FWF (NFN Project “DNTI”, S10703-B03) and the Tiroler Zukunftsstiftung.
Acknowledgment
The authors would like to thank Mrs. Anneliese Niegisch for excellent technical assistance.
References
- Achiwa Y., Hasegawa K., Komiya T., Udagawa Y. Ursolic acid induces Bax-dependent apoptosis through the caspase-3 pathway in endometrial cancer SNG-II cells. Oncology Reports. 2005;13:51–57. [PubMed] [Google Scholar]
- Aguirre-Crespo F., Vergara-Galicia J., Villalobos-Molina R., Javier Lopez-Guerrero J., Navarrete-Vazquez G., Estrada-Soto S. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sciences. 2006;79:1062–1068. doi: 10.1016/j.lfs.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Balanehru S., Nagarajan B. Protective effect of oleanolic acid and ursolic acid against lipid peroxidation. Biochemistry International. 1991;24:981–990. [PubMed] [Google Scholar]
- Bernhard D., Pfister G., Huck C.W., Kind M., Salvenmoser W., Bonn G.K., Wick G. Disruption of vascular endothelial homeostasis by tobacco smoke: impact on atherosclerosis. The FASEB Journal. 2003;17:2302–2304. doi: 10.1096/fj.03-0312fje. [DOI] [PubMed] [Google Scholar]
- Besemer J., Harant H., Wang S., Oberhauser B., Marquardt K., Foster C.A., Schreiner E.P., de Vries J.E., Dascher-Nadel C., Lindley I.J.D. Selective inhibition of cotranslational translocation of vascular cell adhesion molecule 1. Nature. 2005;436:290–293. doi: 10.1038/nature03670. [DOI] [PubMed] [Google Scholar]
- Cardenas C., Quesada A.R., Medina M.A. Effects of ursolic acid on different steps of the angiogenic process. Biochemical and Biophysical Research Communications. 2004;320:402–408. doi: 10.1016/j.bbrc.2004.05.183. [DOI] [PubMed] [Google Scholar]
- Cha H.J., Bae S.K., Lee H.Y., Lee O.H., Sato H., Seiki M., Park B.C., Kim K.W. Anti-invasive activity of ursolic acid correlates with the reduced expression of matrix metalloproteinase-9 (MMP-9) in HT1080 human fibrosarcoma cells. Cancer Research. 1996;56:2281–2284. [PubMed] [Google Scholar]
- Ebrahimzadeh M.A., Mahmoudi M., Salimi E. Antiinflammatory activity of Sambucus ebulus hexane extracts. Fitoterapia. 2006;77:146–148. doi: 10.1016/j.fitote.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Hiermann A. Sambucus. In: Blaschek W., Ebel S., Hackenthal E., Holzgrabe U., Keller K., Reichling J., Schulz V., editors. 6th ed. vol. 14. Wissenschaftliche Verlagsgesellschaft mbH; Stuttgart, Germany: 2007. pp. 173–189. (Hagers Enzyklopädie der Arzneistoffe und Drogen). [Google Scholar]
- Ikeda Y., Murakami A., Ohigashi H. Ursolic acid promotes the release of macrophage migration inhibitory factor via ERK2 activation in resting mouse macrophages. Biochemical Pharmacology. 2005;70:1497–1505. doi: 10.1016/j.bcp.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Murakami A., Ohigashi H. Ursolic acid: an anti- and pro-inflammatory triterpenoid. Molecular Nutrition & Food Research. 2008;52:26–42. doi: 10.1002/mnfr.200700389. [DOI] [PubMed] [Google Scholar]
- Lee H.Y., Chung H.Y., Kim K.H., Lee J.J., Kim K.W. Induction of differentiation in the cultured F9 teratocarcinoma stem cells by triterpene acids. Journal of Cancer Research and Clinical Oncology. 1994;120:513–518. doi: 10.1007/BF01221027. [DOI] [PubMed] [Google Scholar]
- Lu J., Zheng Y.L., Wu D.M., Luo L., Sun D.X., Shan Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by d-galactose. Biochemical Pharmacology. 2007;74:1078–1090. doi: 10.1016/j.bcp.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Nakanishi C., Toi M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nature Reviews Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- Pieri V., Schwaiger S., Ellmerer E.P., Stuppner H. Iridoid glycosides from the leaves of Sambucus ebulus. Journal of Natural Products. 2009;72:1798–1803. doi: 10.1021/np900373u. [DOI] [PubMed] [Google Scholar]
- Ramos A.A., Lima C.F., Pereira M.L., Fernandes-Ferreira M., Pereira-Wilson C. Antigenotoxic effects of quercetin, rutin and ursolic acid on HepG2 cells: evaluation by the comet assay. Toxicology Letters. 2008;177:66–73. doi: 10.1016/j.toxlet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Ramachandran S., Prasad N.R. Effect of ursolic acid, a triterpenoid antioxidant, on ultraviolet-B radiation-induced cytotoxicity, lipid peroxidation and DNA damage in human lymphocytes. Chemico-Biological Interactions. 2008;176:99–107. doi: 10.1016/j.cbi.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Richter W., Wilhun G. Data of the constituents of Sambucus nigra L. III. Determination of ursol and oleanolic acids, amyrin and sterol contents from Sambuci DAB 7 flowers. Pharmazeutische Zeitung. 1977;122:1567–1571. [Google Scholar]
- Serra C., Lampis G., Pompei R., Pinza M. Antiviral activity of new triterpenic derivatives. Pharmacological Research. 1994;29:359–366. doi: 10.1016/1043-6618(94)80057-x. [DOI] [PubMed] [Google Scholar]
- Shishodia S., Majumdar S., Banerjee S., Aggarwal B.B. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Research. 2003;63:4375–4383. [PubMed] [Google Scholar]
- Sohn K.H., Lee H.Y., Chung H.Y., Young H.S., Yi S.Y., Kim K.W. Anti-angiogenic activity of triterpene acids. Cancer Letters. 1995;94:213–218. doi: 10.1016/0304-3835(95)03856-r. [DOI] [PubMed] [Google Scholar]
- Somova L.O., Nadar A., Rammanan P., Shode F.O. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine. 2003;10:115–121. doi: 10.1078/094471103321659807. [DOI] [PubMed] [Google Scholar]
- Steinkamp-Fenske K., Bollinger L., Voller N., Xu H., Yao Y., Bauer R., Förstermann U., Li H. Ursolic acid from the Chinese herb danshen (Salvia miltiorrhiza L.) upregulates eNOS and down regulates Nox4 expression in human endothelial cells. Atherosclerosis. 2007;195:e104–e111. doi: 10.1016/j.atherosclerosis.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K., Michaluart P., Sporn M.B., Dannenberg A.J. Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Research. 2000;60:2399–2404. [PubMed] [Google Scholar]
- Thuong P.T., Jin W.Y., Lee J.P., Seong R.S., Lee Y.M., Seong Y.H., Song K.S., Bae K.H. Inhibitory effect on TNFalpha-induced IL-8 production in the HT29 cell of constituents from the leaf and stem of Weigela subsessilis. Archives of Pharmacal Research. 2005;28:1135–1141. doi: 10.1007/BF02972975. [DOI] [PubMed] [Google Scholar]
- Tundis R., Deguin B., Menichini F., Tillequin F. Iridoids from Putoria calabrica. Biochemical Systematics and Ecology. 2002;30:689–691. [Google Scholar]
- Whiteman S.C., Spiteri M.A. IFN-gamma regulation of ICAM-1 receptors in bronchial epithelial cells: soluble ICAM-1 release inhibits human rhinovirus infection. Journal of Inflammation. 2008;5 doi: 10.1186/1476-9255-5-8. doi:10.1186/1476-9255-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilada E., Üstün O., Sezik E., Takaishi Y., Ono Y., Honda G.J. Inhibitory effects of Turkish folk remedies on inflammatory cytokines: interleukin-1α, interleukin-1β and tumor necrosis factor α. Journal of Ethnopharmacology. 1997;58:59–73. doi: 10.1016/s0378-8741(97)00076-7. [DOI] [PubMed] [Google Scholar]
- Yesilada E. Evaluation of the anti-inflammatory activity of the Turkish medicinal plant Sambucus ebulus. Chemistry of Natural Compounds. 1997;33:539–540. [Google Scholar]
- Zhang Y., Guan L., Wang X., Wen T., Xing J., Zhao J. Protection of chlorophyllin against oxidative damage by inducing HO-1 and NQO1 expression mediated by PI3K/Akt and Nrf2. Free Radical Research. 2008;42:362–371. doi: 10.1080/10715760801993076. [DOI] [PubMed] [Google Scholar]