Abstract
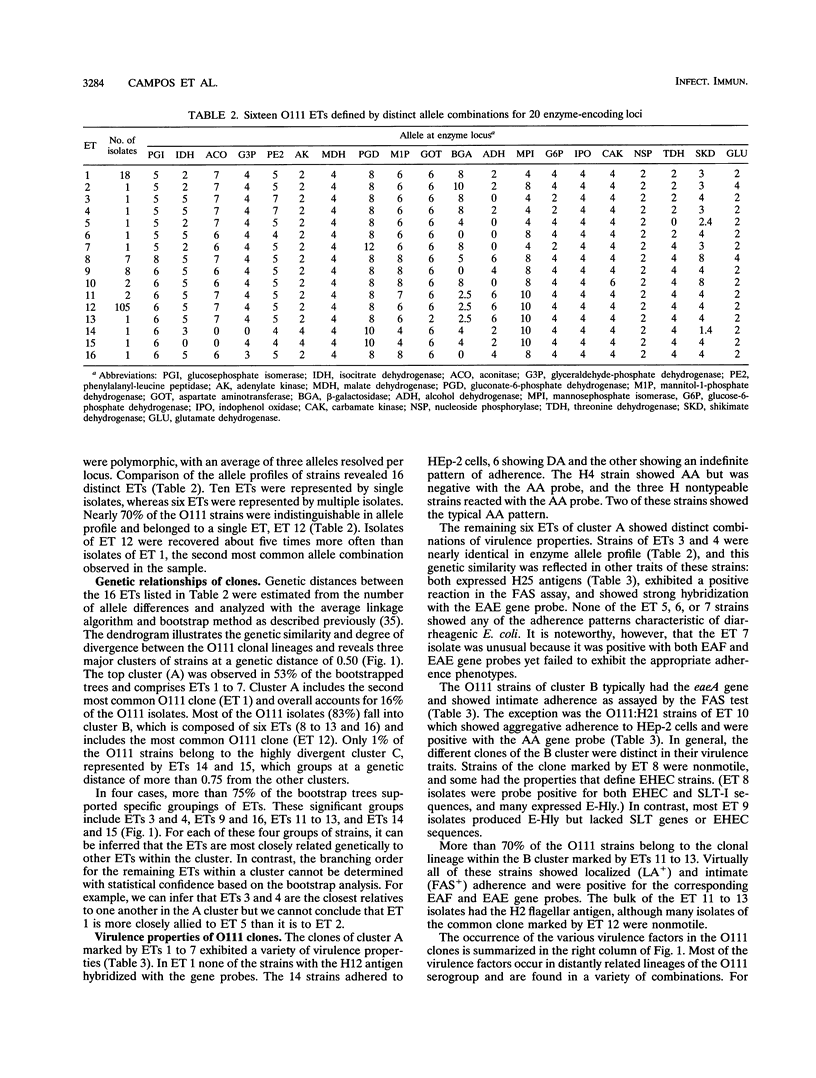
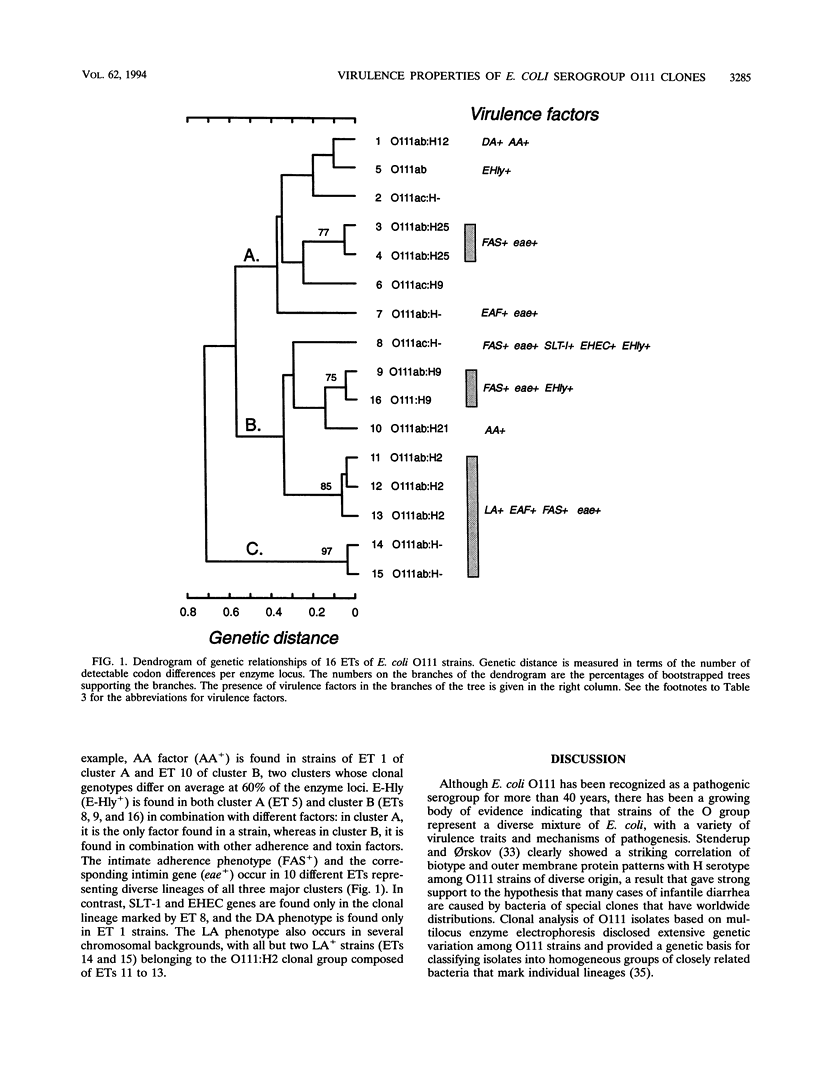
Genetic variation among isolates of Escherichia coli O111 obtained mostly from patients with diarrhea in Brazil was assessed by multilocus enzyme electrophoresis to characterize chromosomal genotypes and by gene probes and adherence assays to characterize virulence properties. Among the 152 isolates, we resolved 16 distinct electrophoretic types (ETs), which differed on average at 40% of the enzyme loci. We identified four major bacterial O111 clones of different disease classes: ET 12, which includes the bulk of the enteropathogenic E. coli strains, typically showing localized adherence and intimate attachment in tissue culture assays; ET 1, which includes strains with a different set of virulence markers; ET 9, which includes strains that show intimate attachment but lack localized adherence and Shiga-like toxin genes; and ET 8, which includes strains that are Shiga-like toxin producers and have the corresponding traits of enterohemorrhagic E. coli. Enteroaggregative strains constituted ET 10 and also occurred in ET 1. Isolates of the major clones were found in South and North America and matched in ET and virulence factors to previously described diarrheagenic clones that are widely disseminated in the human population. Because the major clones are genetically distantly related and exhibit different combinations of virulence factors, we hypothesize that they have distinct mechanisms of pathogenesis. The results indicate that genetic divergence of bacteria with the O111 antigen, as measured by allelic variation in enzyme loci, is accompanied by divergence in virulence properties of clones so that identification and classification of pathogenic E. coli strains cannot be based solely on serotyping or a single virulence factor.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudry B., Savarino S. J., Vial P., Kaper J. B., Levine M. M. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrheal pathogen. J Infect Dis. 1990 Jun;161(6):1249–1251. doi: 10.1093/infdis/161.6.1249. [DOI] [PubMed] [Google Scholar]
- Beutin L., Prada J., Zimmermann S., Stephan R., Orskov I., Orskov F. Enterohemolysin, a new type of hemolysin produced by some strains of enteropathogenic E. coli (EPEC). Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Mar;267(4):576–588. doi: 10.1016/s0176-6724(88)80042-7. [DOI] [PubMed] [Google Scholar]
- Bilge S. S., Clausen C. R., Lau W., Moseley S. L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989 Aug;171(8):4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher-Hennings J., Willgohs J. A., Francis D. H., Raman U. A., Moxley R. A., Hurley D. J. Immunocompromise in gnotobiotic pigs induced by verotoxin-producing Escherichia coli (O111:NM). Infect Immun. 1993 Jun;61(6):2304–2308. doi: 10.1128/iai.61.6.2304-2308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T. A., Vieira M. A., Wachsmuth I. K., Blake P. A., Trabulsi L. R. Serotype-specific prevalence of Escherichia coli strains with EPEC adherence factor genes in infants with and without diarrhea in São Paulo, Brazil. J Infect Dis. 1989 Jul;160(1):131–135. doi: 10.1093/infdis/160.1.131. [DOI] [PubMed] [Google Scholar]
- Gurwith M., Hinde D., Gross R., Rowe B. A prospective study of enteropathogenic Escherichia coli in endemic diarrheal disease. J Infect Dis. 1978 Mar;137(3):292–297. doi: 10.1093/infdis/137.3.292. [DOI] [PubMed] [Google Scholar]
- KAUFFMANN F., DUPONT A. Escherichia strains from infantile epidemic gastro enteritis. Acta Pathol Microbiol Scand. 1950;27(4):552–564. doi: 10.1111/j.1699-0463.1950.tb04927.x. [DOI] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Phillips A. D., Smith H. R., Gross R. J., Shaw R., Watson P., Price E. Screening for enteropathogenic Escherichia coli in infants with diarrhea by the fluorescent-actin staining test. Infect Immun. 1991 Jan;59(1):365–371. doi: 10.1128/iai.59.1.365-371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D. Adhesion and its role in the virulence of enteropathogenic Escherichia coli. Clin Microbiol Rev. 1994 Apr;7(2):152–173. doi: 10.1128/cmr.7.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987 Mar;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Xu J. G., Kaper J. B., Lior H., Prado V., Tall B., Nataro J., Karch H., Wachsmuth K. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1987 Jul;156(1):175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- Maas R. An improved colony hybridization method with significantly increased sensitivity for detection of single genes. Plasmid. 1983 Nov;10(3):296–298. doi: 10.1016/0147-619x(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Moyenuddin M., Wachsmuth I. K., Moseley S. L., Bopp C. A., Blake P. A. Serotype, antimicrobial resistance, and adherence properties of Escherichia coli strains associated with outbreaks of diarrheal illness in children in the United States. J Clin Microbiol. 1989 Oct;27(10):2234–2239. doi: 10.1128/jcm.27.10.2234-2239.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro J. P., Baldini M. M., Kaper J. B., Black R. E., Bravo N., Levine M. M. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J Infect Dis. 1985 Sep;152(3):560–565. doi: 10.1093/infdis/152.3.560. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Kaper J. B., Robins-Browne R., Prado V., Vial P., Levine M. M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987 Sep;6(9):829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Scaletsky I. C., Kaper J. B., Levine M. M., Trabulsi L. R. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect Immun. 1985 May;48(2):378–383. doi: 10.1128/iai.48.2.378-383.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland J. W., Neill R. J. DNA probes for Shiga-like toxins I and II and for toxin-converting bacteriophages. J Clin Microbiol. 1988 Jul;26(7):1292–1297. doi: 10.1128/jcm.26.7.1292-1297.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov F., Orskov I. Escherichia coli serotyping and disease in man and animals. Can J Microbiol. 1992 Jul;38(7):699–704. [PubMed] [Google Scholar]
- Orskov F., Whittam T. S., Cravioto A., Orskov I. Clonal relationships among classic enteropathogenic Escherichia coli (EPEC) belong to different O groups. J Infect Dis. 1990 Jul;162(1):76–81. doi: 10.1093/infdis/162.1.76. [DOI] [PubMed] [Google Scholar]
- Paulozzi L. J., Johnson K. E., Kamahele L. M., Clausen C. R., Riley L. W., Helgerson S. D. Diarrhea associated with adherent enteropathogenic Escherichia coli in an infant and toddler center, Seattle, Washington. Pediatrics. 1986 Mar;77(3):296–300. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Yam W. C., O'Gorman L. E., Bettelheim K. A. Examination of archetypal strains of enteropathogenic Escherichia coli for properties associated with bacterial virulence. J Med Microbiol. 1993 Mar;38(3):222–226. doi: 10.1099/00222615-38-3-222. [DOI] [PubMed] [Google Scholar]
- Rosenshine I., Donnenberg M. S., Kaper J. B., Finlay B. B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992 Oct;11(10):3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaletsky I. C., Silva M. L., Toledo M. R., Davis B. R., Blake P. A., Trabulsi L. R. Correlation between adherence to HeLa cells and serogroups, serotypes, and bioserotypes of Escherichia coli. Infect Immun. 1985 Sep;49(3):528–532. doi: 10.1128/iai.49.3.528-532.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaletsky I. C., Silva M. L., Trabulsi L. R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984 Aug;45(2):534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland S. M., Smith H. R., Rowe B. Escherichia coli O128 strains from infants with diarrhea commonly show localized adhesion and positivity in the fluorescent-actin staining test but do not hybridize with an enteropathogenic E. coli adherence factor probe. Infect Immun. 1991 Apr;59(4):1569–1571. doi: 10.1128/iai.59.4.1569-1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenderup J., Orskov F. The clonal nature of enteropathogenic Escherichia coli strains. J Infect Dis. 1983 Dec;148(6):1019–1024. doi: 10.1093/infdis/148.6.1019. [DOI] [PubMed] [Google Scholar]
- Viljanen M. K., Peltola T., Junnila S. Y., Olkkonen L., Järvinen H., Kuistila M., Huovinen P. Outbreak of diarrhoea due to Escherichia coli O111:B4 in schoolchildren and adults: association of Vi antigen-like reactivity. Lancet. 1990 Oct 6;336(8719):831–834. doi: 10.1016/0140-6736(90)92337-h. [DOI] [PubMed] [Google Scholar]
- Whittam T. S., Wolfe M. L., Wachsmuth I. K., Orskov F., Orskov I., Wilson R. A. Clonal relationships among Escherichia coli strains that cause hemorrhagic colitis and infantile diarrhea. Infect Immun. 1993 May;61(5):1619–1629. doi: 10.1128/iai.61.5.1619-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


