Abstract
Oeosinophilic gastroenteritis is a chronic and rare disorder characterised by massive oeosinophilic tissue infiltration involving one or more segments of the digestive tract. The management of patients with oeosinophilic gastroenteritis is complex and the therapeutic response often poor. Here we discuss the clinical case and management of a 23-year-old man with oeosinophilic gastroenteritis since the first year of life and the decision to prescribe lifelong total parenteral nutrition.
Background
Oeosinophilic gastroenteritis is a chronic, rare, disorder affecting predominantly men characterised by an oeosinophil-predominant inflammatory process involving one or more segments of the digestive tract.1
Its estimated incidence is 1–20 individuals out of every 100 000, although many cases are under-diagnosed or not reported.2 Oeosinophilic gastroenteritis has been largely described in the paediatric population and in adults it is most often detected during endoscopic investigation for abdominal pain or diarrhoea.3
The inflammatory process may involve one or more areas of the gastrointestinal tract from the oesophagus to the colon, the liver, the biliary tract, the pancreas and the peritoneum.4–7 The clinical manifestations depend on the extent, location and depth of the oeosinophilic inflammatory infiltration within the gastrointestinal tract.1 2
In particular, mucosal involvement is typically associated with nausea, vomiting, diarrhoea, abdominal pain, occult or frank bleeding with iron deficiency, weight loss secondary to malabsorption and protein-losing enteropathy; the involvement of the muscular layers could lead to intestinal obstruction or even acute abdomen. Finally, serosal involvement can cause bloating and ascites.8–10
Oeosinophilic gastroenteritis can be associated with peripheral blood oeosinophilia, elevated serum IgE levels and food-related hypersensitivity reactions, sometimes with systemic oeosinophilic disorders.11
The diagnosis of oeosinophilic gastroenteritis is based on the following criteria: (1) gastrointestinal symptoms, (2) oeosinophilic infiltration of one or more areas of the gastrointestinal tract and (3) exclusion of other causes for intestinal oeosinophilia (eg, drug reactions, parasitic infections, inflammatory bowel diseases, connective tissue diseases, lymphoproliferative malignancies).12 13
The disease is often characterised by insensitiveness to different treatments and, consequently, the management is very complex.
Here, we describe the clinical case of a young man with oeosinophilic gastroenteritis associated with multiple intestinal fistulas and stenosis, relapsing pneumonia and severe otitis, nasal polyposis, T cell immunodeficiency and multiple food allergies.
Case presentation
On November 2006, a 20-year-old man with oeosinophilic gastroenteritis was admitted to our ward due to pneumonia and severe protein-calorie malnutrition after partial gastrectomy, duodenectomy and cholecistectomy performed 15 days before to treat a bleeding pyloric ulcer.
The patient's familial anamnesis included the premature death of a 2-year-old sister for pneumonia and diarrhoea. At 2, 6 and 12 months of life he had episodes of pneumonia and diarrhoea and was diagnosed with T cell immunodeficiency and selective deficit of T cell helper lymphocytes.
He had reactions to many food allergens, and the attempt to introduce two selected foods that had been negative at the cutaneous prick tests resulted in vomiting, abdominal pain, diarrhoea and increased intestinal permeability. Therefore, he could not have a natural diet and was fed with an elementary diet since the first year of life.
At the age of 5 years, he was again admitted to hospital for abdominal pain. An upper gastrointestinal endoscopy showed gastrointestinal mucosa hyperaemia with erosions and ulcerations; the antropyloric region was obstructed and an oeosinophilic inflammatory infiltration was found in all biopsies. Repeated sweat tests were always negative as were the serum anti-transglutaminase and anti-endomisium antibodies; the α1-antitripsine and total immunoglobulin levels were in the normal range. He was diagnosed as having oeosinophilic gastroenteritis although steroid treatment (2 mg/kg/day for the first week and 0.5 mg/kg/day for the following 8 weeks) did not induce satisfying results14–17; finally, enteral nutrition with an elemental diet through a nasogastric tube (NGT) was prescribed with satisfying effects on the disease symptoms.
At 6 years of age, he had Nissen anti-reflux surgery and a percutaneous endoscopic gastrostomy (PEG) positioning to continue the enteral nutrition with the elemental diet. During these years, growth rate was poor and recurring episodes of pneumonia and otitis were recorded.
Faringeal and sputum swabs were often positive for Staphylococcus aureus and Pseudomonas aeuruginosa. Laboratory data showed high IgE levels.
An upper endoscopy revealed a stenosis of the gastric antrum with oedematous and weak mucosa, but the probe could not go beyond the pylorus. The histology showed a chronic inflammatory infiltrate with a high percentage of oeosinophils. The iron load test was pathological, while gut permeability tests with lactulose and mannitol were within the normal range. The intestinal washing content showed a total immunoglobulin level at 12 mg/ml (nv<5) and an increased haemoglobin concentration.
At 10 years of age, he experienced gastric bleeding and an upper endoscopy showed a prepyloric ulcer with complete pylorus obstruction. He underwent pyloroplasty and the histology of the biopsy samples confirmed an increased number of oeosinophils.
At 20 years of age (19 days before admission to our hospital), he was urgently admitted for hypovolemic shock due to a bleeding pyloric ulcer; after several attempts of endoscopic haemostasis, he underwent gastroduodenal resection and cholecystectomy for dropsied cholecystis as already reported. The histological examination of the surgically resected bowel segment showed oedema, hyperaemia and lympho-plasmocitary infiltration of mucosa, hypertrophy and oedema of the muscolaris layer, and fibrosis with heavy oeosinophilic infiltration and numerous degranulated oeosinophils in all intestinal wall layers.
A few days later, he was again operated for choleperitoneum due to a gastric fistula on the PEG. Therefore, the PEG was removed and the perforations sutured but an entero-cutaneous fistula opened a few days later.
Investigations
On admission to our ward, the patient was dyspnoic and very malnourished.
A chest x-ray showed a bronchopneumonic process with diffuse thickening of bronchial tubes. The Koch Bacillus search on blood, urine and sputum was negative. Large spectrum antibiotic treatment was started.
Protein-energy malnutrition indexes, such as reduced body weight of 36 kg, reduced body mass index (BMI) of 14.1 kg/m2, reduced resting metabolic rate (RMR) 563 kcal/24 h and reduced bioelectrical impedance (BIA) phase angle 4.3 (nv 6–7 for men) resulted.18
Serum ferritin values were 971 ng/dl (nv 18–370), total IgE 473 kU/l (nv<100) and parathyroid hormone 6 pg/ml (nv 10–75). Hydrosoluble and liposoluble vitamin serum levels were found very low.
After adequate desensitising premedication, an intestinal x-ray with gastrographyn through the NGT was performed (figure 1), which showed a progressive iodinate contrast accumulation in the resected gastric cavity with only a slow flow to the gastro-jejunostomy and stagnation in the afferent loop. Moreover, several reflux episodes and the intestinal lumen obstruction were also shown.
Figure 1.
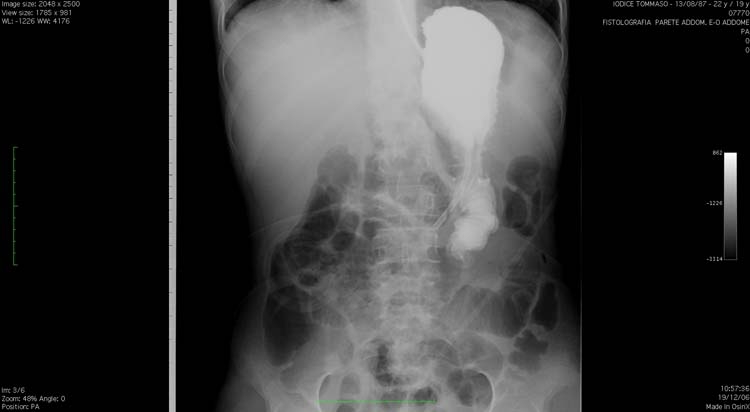
Intestinal x-ray with gastrographyn through the nasogastric tube showing the contrast accumulation in the resected gastric cavity and afferent loop, with only a slow flow to the gastro-jejunostomy.
About 8 h after the test, the patient complained of severe abdominal pain; the abdominal direct x-ray performed showed multiple hydro-aereus levels and a CT scan revealed a fluid collection in the abdominal recesses, with gaseous inclusions among the ileal loops, which were restricted and cleaved.
Treatment
The NGT was removed and a temporary central venous catheter (CVC) was positioned for parenteral nutrition.
When the clinical conditions improved and the antibiotic treatment for pneumonia was completed, it was decided to position a long-term tunnelled CVC to manage lifelong total parenteral nutrition.
Following a gradual and careful nutritional rehabilitation, a full calorie nutritional mixture (2000 kcal; amino acids 85 g; glucose 250 g; Medium Chain Triglycerides/Long Chain Triglycerides (MCT/LCT) lipids 70 g) with the addition of multivitamins and micronutrients was prescribed; moreover, the patient received additional parenteral vitamin supplementations (folic acid, A, B1, B6, B12, D, E, K vitamins) in order to restore these vitamin and micronutrient normal levels.
Outcome and follow-up
At discharge, he was prescribed multiple drug treatment with low-dose corticosteroid and antihistaminic agents to complete the treatment of pneumonia and cyclic teicoplanin administration to prevent respiratory and ear infections in addition to total parenteral nutrition. The patient and his relatives were carefully instructed on the proper management of the CVC.
His clinical conditions progressively improved. At the most recent follow-up, 36 months after the total parenteral nutrition, his clinical conditions were satisfying and stable. His body weight was 42 kg and BMI 15.3 kg/m2, BIA phase angle 6.7 and RMR 1541 kcal/24 h due to the gradual restoration of lean body mass. There was no evidence of iron deficit and nor had he experienced relapses of respiratory infection. His quality of life is now satisfactory; he receives cyclic nocturnal home parenteral nutrition every day, works as accountant and is able to drive a car. He performs periodical outpatient checkups at our outpatient unit.
Discussion
Primary oeosinophilic gastroenteritis comprises a pool of uncommon and heterogeneous disorders secondary to a prominent oeosinophilic tissue infiltration of the gastrointestinal tract of as yet unknown origin.2 3 11
The main aspect of oeosinophilic gastroenteritis is the often inconstant and insufficient response to several treatments. However in this case report, we showed as total parenteral nutrition has been able to dramatically improve the patient's clinical conditions.19 20
Our case study reports on a patient with T cell immunodeficiency in whom the defective peripheral tolerance to an intestine-specific autoantigen probably led to an uncontrolled inflammation of the intestinal wall with a conspicuous oeosinophilic infiltrate.19 20
Indeed, according to the data reported in the literature, the initial diagnosis (T cell immunodeficiency) has to be considered part of the oeosinophilic gastroenteritis syndrome; rather, it is possible that the underlying disease is an immunodeficiency and oeosinophilic gastroenteritis is a manifestation.
In fact, in several human conditions associated with limited thymic output of T cells, striking immunopathology occurs usually associated with oeosinophilic inflammation and with the production of Th2 cytokines. In these individuals, the repertoire of T cell receptors expressed by peripheral T cells results are often very limited and this seems to be the main factor determining the uncontrolled CD4 T cell proliferation.19 20
Finally, a case report of a 34-year-old woman with disordered IgE antibody regulation as a consequence of HIV-1 infection (acquired immunodeficiency) has been described.21
Since the age of 1 year, due to intolerance to multiple foods, our patient received an elemental diet and the attempt to introduce two selected foods that had been negative at the cutaneous prick test resulted in severe gastrointestinal symptoms.
Steroidal treatment and elemental diet were not successful in controlling the evolution of the disease; therefore, with time, besides the poor clinical conditions, the patient's general status required three surgical operations to treat the ensuing obstructive complications.22
The patient experienced an ileus after x-ray was performed with soluble contrast due to the multiple intestinal stenosis for the spread of the disease; this event confirmed that the patient could not be fed with enteral nutrition.
In analogy with chronic idiopathic intestinal pseudobstruction, where patients cannot be fed orally or via an enteral tube, total parenteral nutrition through a long-term CVC appeared, in our opinion, the only possible treatment in this specific case of multiple stenosis of the gastroenteric tract with severe malnutrition.22 23
In the meantime, the efficacy of this treatment has been confirmed by the interruption of the gastric, infectious and metabolic complications as well by the stable improvement of the patient's nutritional state.
Home parenteral nutrition complications could be metabolic, mechanical and infective; their rate is generally low and strongly depends on the correct trade-off of nutritional mixture components and the careful CVC management (by the nurses, the patient and the caregivers). Data in the literature report a CVC infection rate of 2–0.3/1000 catheter days, while the thrombosis and occlusion rate is 0.02/1000 days of treatment.
During the 3 years of follow-up, this patient had no home parenteral nutrition/CVC complications.24–26
Learning points.
-
▶
This case report describes the troublesome management of a complex and rare disease, contributing to the debate on its correct handling.
-
▶
Oeosinophilic gastroenteritis can compromise food intake and digestion causing severe malnutrition.
-
▶
This disease is often drug resistant and can require surgical intestinal resections or other “non-standard” therapies.
-
▶
The consultation of different specialists has allowed the best therapeutic decisions to be made in order to guarantee a good quality of life for the patient.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Talley NJ, Shorter RG, Phillips SF, et al. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut 1990;31:54–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Straumann A. Idiopathic eosinophilic gastrointestinal diseases in adults. Best Pract Res Clin Gastroenterol 2008;22:481–96 [DOI] [PubMed] [Google Scholar]
- 3.Khan S, Orenstein SR. Eosinophilic gastroenteritis. Gastroenterol Clin North Am 2008;37:333–48, v [DOI] [PubMed] [Google Scholar]
- 4.Straumann A, Rossi L, Simon HU, et al. Fragility of the esophageal mucosa: a pathognomonic endoscopic sign of primary eosinophilic esophagitis? Gastrointest Endosc 2003;57:407–12 [DOI] [PubMed] [Google Scholar]
- 5.Flejou JF, Potet F, Bernades P. [Eosinophilic pancreatitis: a rare manifestation of digestive allergy?]. Gastroenterol Clin Biol 1989;13:731–3 [PubMed] [Google Scholar]
- 6.Dabbs DJ. Eosinophilic and lymphoeosinophilic cholecystitis. Am J Surg Pathol 1993;17:497–501 [DOI] [PubMed] [Google Scholar]
- 7.Moore D, Lichtman S, Lentz J, et al. Eosinophilic gastroenteritis presenting in an adolescent with isolated colonic involvement. Gut 1986;27:1219–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yun MY, Cho YU, Park IS, et al. Eosinophilic gastroenteritis presenting as small bowel obstruction: a case report and review of the literature. World J Gastroenterol 2007;13:1758–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheikh RA, Prindiville TW, Pecha RE, et al. Unusual presentations of eosinophilic gastroenteritis: Case series and review of literature. World J Gastroenterol 2009;15:2156–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendez-Sanchez N, Chavez-Tapia NC, Vazquez-Elizondo G, et al. Eosinophilic gastroenteritis: a review. Dig Dis Sci 2007;52:2904–11 [DOI] [PubMed] [Google Scholar]
- 11.Straumann A, Simon HU. The physiological and pathophysiological roles of eosinophils in the gastrointestinal tract. Allergy 2004;59:15–25 [DOI] [PubMed] [Google Scholar]
- 12.Freeman HJ. Adult eosinophilic gastroenteritis and hypereosinophilic syndromes. World J Gastroenterol 2008;14:6771–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh HE, Chetty R. Eosinophilic gastroenteritis: a review. J Gastroenterol 2008;43:741–750 [DOI] [PubMed] [Google Scholar]
- 14.Straumann A, Spichtin HP, Simon HU, et al. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 12 years [abstract]. Gastroenterol 2003;124:M874. [DOI] [PubMed] [Google Scholar]
- 15.Hümmer-Ehret BH, Rohrschneider WK, Oleszczuk-Raschke K, et al. Eosinophilic gastroenteritis mimicking idiopathic hypertrophic pyloric stenosis. Pediatr Radiol 1998;28:711–13 [DOI] [PubMed] [Google Scholar]
- 16.Aquino A, Dòmini M, Rossi C, et al. Pyloric stenosis due to eosinophilic gastroenteritis: presentation of two cases in mono-ovular twins. Eur J Pediatr 1999;158:172–3 [DOI] [PubMed] [Google Scholar]
- 17.Tursi A, Rella G, Inchingolo CD, et al. Gastric outlet obstruction due to gastroduodenal eosinophilic gastroenteritis. Endoscopy 2007;39(Suppl 1):E184. [DOI] [PubMed] [Google Scholar]
- 18.ASPEN Board of Directors and the Clinical Guidelines Task Force Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26(1 Suppl):1SA–138SA Erratum in: JPEN J Parenter Enteral Nutr 2002;26:44 [PubMed] [Google Scholar]
- 19.Cousins L, Graham M, Tooze R, et al. Eosinophilic bowel disease controlled by the BB rat-derived lymphopenia/Gimap5 gene. Gastroenterology 2006;131:1475–85 [DOI] [PubMed] [Google Scholar]
- 20.Stefenelli N, Leibl W, Brandstetter G, et al. [Agammaglobulinemia and eosinophilic gastroenteritis]. Wien Klin Wochenschr 1980;92:840–4 [PubMed] [Google Scholar]
- 21.Mazza DS, O'Sullivan M, Grieco MH. HIV-1 infection complicated by food allergy and allergic gastroenteritis: a case report. Ann Allergy 1991;66:436–40 [PubMed] [Google Scholar]
- 22.Lefebvre C, Bletry O, Degoulet P, et al. [Prognostic factors of hypereosinophilic syndrome. Study of 40 cases]. Ann Med Interne (Paris) 1989;140:253–7 [PubMed] [Google Scholar]
- 23.Mann SD, Debinski HS, Kamm MA. Clinical characteristics of chronic idiopathic intestinal pseudo-obstruction in adults. Gut 1997;41:675–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bozzetti F, Mariani L, Bertinet DB, et al. Central venous catheter complications in 447 patients on home parenteral nutrition: an analysis of over 100.000 catheter days. Clin Nutr 2002;21:475–85 [DOI] [PubMed] [Google Scholar]
- 25.Santarpia L, Alfonsi L, Tiseo D, et al. Central venous catheter infections and antibiotic therapy during long-term home parenteral nutrition: an 11-year follow-up study. JPEN J Parenter Enteral Nutr 2010;34:254–62 [DOI] [PubMed] [Google Scholar]
- 26.Nishinari K, Wolosker N, Bernardi CV, et al. Totally implantable ports connected to valved catheters for chemotherapy: experience from 350 Groshong devices. J Vasc Access 2010;11:17–22 [DOI] [PubMed] [Google Scholar]


