Abstract
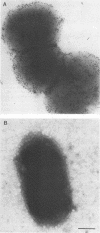
Actinobacillus pleuropneumoniae is the causative agent of porcine pleuropneumonia. The major adhesin of A. pleuropneumoniae has been identified as the lipopolysaccharides (LPSs) (M. Bélanger, D. Dubreuil, J. Harel, C. Girard, and M. Jacques, Infect. Immun. 58:3523-3530, 1990). Using immunoelectron microscopy and flow cytometry, we showed in the present study that LPSs were well exposed at the surface of this encapsulated microorganism. Immunolocalization with porcine lung and tracheal frozen sections showed that extracted LPS bound to the lung mesenchyme and vascular endothelium and to the tracheal epithelium, respectively. Inhibition of adherence of A. pleuropneumoniae with extracted LPS was also performed with lung and tracheal frozen sections. Acid hydrolysis of LPS revealed that the active component of LPS was not lipid A but the polysaccharides. LPSs from A. pleuropneumoniae serotypes 1 and 2 were separated by chromatography on Sephacryl S-300 SF, in the presence of sodium deoxycholate, according to their molecular masses. The adherence-inhibitory activity was found in the high-molecular-mass fractions. These high-molecular-mass fractions contained 2-keto-3-deoxyoctulosonic acid and neutral sugars, and they were recognized by a monoclonal antibody directed against A. pleuropneumoniae O antigen but not recognized by a monoclonal antibody against capsular antigen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bada Alambedji R., Dubreuil J. D. Comparaison de deux antigènes de nature polysaccharidique pour le diagnostic sérologique par ELISA de la pleuropneumonie porcine (sérotype 5). Zentralbl Veterinarmed B. 1993 Jun;40(4):253–260. [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Brade H., Brade L., Rietschel E. T. Structure-activity relationships of bacterial lipopolysaccharides (endotoxins). Current and future aspects. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):151–179. doi: 10.1016/s0176-6724(88)80001-4. [DOI] [PubMed] [Google Scholar]
- Broes A., Fairbrother J. M., Larivière S., Jacques M., Johnson W. M. Virulence properties of enterotoxigenic Escherichia coli O8: KX105 strains isolated from diarrheic piglets. Infect Immun. 1988 Jan;56(1):241–246. doi: 10.1128/iai.56.1.241-246.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd W., Kadis S. Structures and sugar compositions of lipopolysaccharides isolated from seven Actinobacillus pleuropneumoniae serotypes. Infect Immun. 1989 Dec;57(12):3901–3906. doi: 10.1128/iai.57.12.3901-3906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M., Dubreuil D., Harel J., Girard C., Jacques M. Role of lipopolysaccharides in adherence of Actinobacillus pleuropneumoniae to porcine tracheal rings. Infect Immun. 1990 Nov;58(11):3523–3530. doi: 10.1128/iai.58.11.3523-3530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M., Rioux S., Foiry B., Jacques M. Affinity for porcine respiratory tract mucus is found in some isolates of Actinobacillus pleuropneumoniae. FEMS Microbiol Lett. 1992 Oct 1;76(1-2):119–125. doi: 10.1111/j.1574-6968.1992.tb05450.x. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Beck M., Stucki U., Nicolet J. Analysis of hemolysin operons in Actinobacillus pleuropneumoniae. Gene. 1993 Jan 15;123(1):51–58. doi: 10.1016/0378-1119(93)90538-e. [DOI] [PubMed] [Google Scholar]
- Frey J., Bosse J. T., Chang Y. F., Cullen J. M., Fenwick B., Gerlach G. F., Gygi D., Haesebrouck F., Inzana T. J., Jansen R. Actinobacillus pleuropneumoniae RTX-toxins: uniform designation of haemolysins, cytolysins, pleurotoxin and their genes. J Gen Microbiol. 1993 Aug;139(8):1723–1728. doi: 10.1099/00221287-139-8-1723. [DOI] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Hemolysin patterns of Actinobacillus pleuropneumoniae. J Clin Microbiol. 1990 Feb;28(2):232–236. doi: 10.1128/jcm.28.2.232-236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese S. B., Stenbaek E., Nielsen R. Identification of Actinobacillus pleuropneumoniae serotype 2 by monoclonal or polyclonal antibodies in latex agglutination tests. Acta Vet Scand. 1993;34(2):223–225. doi: 10.1186/BF03548215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Cavaillon J. M., Etievant M., Lebbar S., Szabo L. Specific binding of endotoxin to human monocytes and mouse macrophages: serum requirement. Cell Immunol. 1985 Mar;91(1):119–131. doi: 10.1016/0008-8749(85)90037-1. [DOI] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Chaby R., Cavaillon J. M., Szabó L. Lipopolysaccharide receptor on rabbit peritoneal macrophages. I. Binding characteristics. J Immunol. 1982 May;128(5):1950–1954. [PubMed] [Google Scholar]
- Hitchcock P. J., Leive L., Mäkelä P. H., Rietschel E. T., Strittmatter W., Morrison D. C. Lipopolysaccharide nomenclature--past, present, and future. J Bacteriol. 1986 Jun;166(3):699–705. doi: 10.1128/jb.166.3.699-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepelman A. I., Tuomanen E. I. Consequences of microbial attachment: directing host cell functions with adhesins. Infect Immun. 1992 May;60(5):1729–1733. doi: 10.1128/iai.60.5.1729-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques M., Bélanger M., Roy G., Foiry B. Adherence of Actinobacillus pleuropneumoniae to porcine tracheal epithelial cells and frozen lung sections. Vet Microbiol. 1991 Apr;27(2):133–143. doi: 10.1016/0378-1135(91)90004-y. [DOI] [PubMed] [Google Scholar]
- Jacques M., Foiry B., Higgins R., Mittal K. R. Electron microscopic examination of capsular material from various serotypes of Actinobacillus pleuropneumoniae. J Bacteriol. 1988 Jul;170(7):3314–3318. doi: 10.1128/jb.170.7.3314-3318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp E. M., Popma J. K., Anakotta J., Smits M. A. Identification of hemolytic and cytotoxic proteins of Actinobacillus pleuropneumoniae by use of monoclonal antibodies. Infect Immun. 1991 Sep;59(9):3079–3085. doi: 10.1128/iai.59.9.3079-3085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Streck H., Minner I., Krammer P. H., Ruschmann E. Selection of bacterial mutants from Salmonella specifically recognizing determinants on the cell surface of activated T lymphocytes. A novel system to define cell surface structures. Eur J Immunol. 1980 Sep;10(9):685–693. doi: 10.1002/eji.1830100906. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Tobias P. S., Wolfson E., Ulevitch R. J. Plasma lipopolysaccharide (LPS)-binding protein. A key component in macrophage recognition of gram-negative LPS. J Immunol. 1992 Jul 1;149(1):200–206. [PubMed] [Google Scholar]
- Mittal K. R., Higgins R., Larivière S., Nadeau M. Serological characterization of Actinobacillus pleuropneumoniae strains isolated from pigs in Quebec. Vet Microbiol. 1992 Sep;32(2):135–148. doi: 10.1016/0378-1135(92)90101-x. [DOI] [PubMed] [Google Scholar]
- Nielsen R. Serological characterization of Actinobacillus pleuropneumoniae strains and proposal of a new serotype: serotype 12. Acta Vet Scand. 1986;27(3):453–455. doi: 10.1186/BF03548158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Bacterial adherence. Adv Intern Med. 1980;25:503–532. [PubMed] [Google Scholar]
- Peterson A. A., Haug A., McGroarty E. J. Physical properties of short- and long-O-antigen-containing fractions of lipopolysaccharide from Escherichia coli 0111:B4. J Bacteriol. 1986 Jan;165(1):116–122. doi: 10.1128/jb.165.1.116-122.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. A., McGroarty E. J. High-molecular-weight components in lipopolysaccharides of Salmonella typhimurium, Salmonella minnesota, and Escherichia coli. J Bacteriol. 1985 May;162(2):738–745. doi: 10.1128/jb.162.2.738-745.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Kirikae T., Schade F. U., Ulmer A. J., Holst O., Brade H., Schmidt G., Mamat U., Grimmecke H. D., Kusumoto S. The chemical structure of bacterial endotoxin in relation to bioactivity. Immunobiology. 1993 Apr;187(3-5):169–190. doi: 10.1016/S0171-2985(11)80338-4. [DOI] [PubMed] [Google Scholar]
- Tomás J. M., Camprubi S., Merino S., Davey M. R., Williams P. Surface exposure of O1 serotype lipopolysaccharide in Klebsiella pneumoniae strains expressing different K antigens. Infect Immun. 1991 Jun;59(6):2006–2011. doi: 10.1128/iai.59.6.2006-2011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wright S. D. Multiple receptors for endotoxin. Curr Opin Immunol. 1991 Feb;3(1):83–90. doi: 10.1016/0952-7915(91)90082-c. [DOI] [PubMed] [Google Scholar]