Abstract
Defects in interferon-γ axis have been shown to be associated with disseminated mycobacterial disease. A case of a previously healthy HIV seronegative child with disseminated extensively drug resistant Mycobacterium tuberculosis infection presenting as splenic abscesses with lymphadenopathy and progressing to disseminated tuberculosis with absent interferon-γ and interleukin-12 production is reported here.
BACKGROUND
Mycobacterial infections are an important cause of human morbidity and mortality worldwide, more so in developing countries like India. Interferon-γ (IFN-γ) is an important mediator in the cell mediated immunity against mycobacteria and defects in IFN-γ/interleukin-12 (IL-12) axis are known to predispose individuals to mycobacteria.1 Worldwide, the incidence of multidrug resistant tuberculosis is increasing alarmingly. Similarly, tuberculous splenic abscesses, as an initial presentation, are rare in an immunocompetent child. We report a case of extensively drug resistant tuberculosis (TB), presenting as splenic abscesses, with absent IFN-γ and IL-12 production.
CASE PRESENTATION
A 7-year-old boy from a good socioeconomic background had been unwell for almost a year with low grade evening rise fever. In the preceding 3 months he had experienced mild upper abdominal pain with loss of appetite. There was no contact with TB. He was treated with an anti-tubercular treatment (ATT) triple drug regimen comprising isoniazid, rifampicin and ethambutol for 9 months based on a positive Mantoux test and mediastinal adenopathy on chest x ray, although the treatment was not in concordance with the revised national tuberculosis control programme (RNTCP) guidelines in India. Lack of an adequate therapeutic response despite compliance to treatment prompted a referral to our centre. At presentation the child was febrile. A BCG scar was present. He had bilateral axillary lymphadenopathy, upper abdominal tenderness and mild splenomegaly. Screening of the family members and contacts by Mantoux test, chest x ray and multiple sputum acid fast bacilli (AFB) staining and cultures did not reveal any evidence of TB. Non-response to standard ATT necessitated the initiation of category II treatment (2 months of intensive therapy with isoniazid, rifampicin, pyrazinamide, etambutol and streptomycin followed by 5 months continuation therapy with isoniazid, rifampicin and ethambutol as per the RNTCP guidelines in India).
After 2 weeks of treatment the child developed left sided pleural effusion. Although the child improved symptomatically, he developed fever, headache, lethargy and loss of appetite after 4 months. There was bilateral axillary lymphadenopathy, moderate hepatosplenomegaly with restricted abduction and proptosis of the right eye. During his stay in hospital the child developed decorticate posturing, left focal seizures and bilateral papilloedema. He also developed hyponatraemia and raised urine osmolarity. These features were consistent with the syndrome of inappropriate antidiuretic hormone which improved with therapy.
INVESTIGATIONS
The Mantoux test was strongly positive (20×25 mm). Chest x ray revealed mediastinal adenopathy, and abdominal ultrasound revealed multiple hypoechoic/anaechoic lesions in the spleen with a 3.0 cm cystic lesion at the splenic hilum. Computed tomography confirmed the findings of the chest x ray and abdominal ultrasound. Ultrasound guided fine needle aspiration cytology (FNAC) of the mediastinal and splenic lesions showed epitheloid cell granulomas in the background of necrosis with stain positive for AFB. Mycobacterial cultures on Lowenstein–Jensen (LJ) medium showed Mycobacterium tuberculosis at 6 weeks which confirmed the diagnosis of tuberculosis. Gastric aspirates and FNAC of axillary nodes were negative for AFB and mycobacterial cultures on LJ medium from these microbiological specimens were sterile at 8 weeks.
Pleural tap (performed in view of newly diagnosed pleural effusion) at 2 weeks of treatment showed no cells, smear negative for AFB and adenosine deaminase of 105 u/l. Mediastinal lymph node aspirate done during the second hospital admission after 4 months of treatment revealed M tuberculosis on LJ culture medium at 6 weeks. Antibiogram, obtained using the drug proportion method, showed that the organism was resistant to rifampicin, isoniazid, streptomycin and ethambutol. Repeated abdominal ultrasounds revealed no change in the morphology of splenic lesions. Ultrasound guided FNAC of the splenic abscess showed persistence of AFB at 4 months of treatment. Magnetic resonance imaging (MRI) of the brain showed meningitis with tuberculomas in left parietal lobe and right cerebellar hemisphere with infarction in the right parietal region. The orbital MRI and the optic nerves were within normal limits.
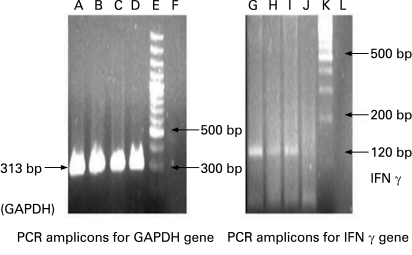
Estimation of IFN-γ and IL-12 concentrations in the child was done using semiquantitative reverse transcriptase polymerase chain reaction technique (RT-PCR), which showed absent production (fig 1). Retroviral serology was negative. Nitroblue tetrazolium test was normally reactive and immunoglobulin values were normal.
Figure 1. Polymerase chain reaction (PCR) amplicons from lymphocytes using semi-quantitative reverse transcriptase PCR.
The study patient is negative for interferon-γ (IFN-γ) mRNA expression (lane J) while other patient samples are positive for IFN-γ mRNA expression. Lanes A–C, G–I: positive controls. D, J: study patient; E, K: molecular weight 100 bp markers; F, L: negative controls.
TREATMENT
In view of disseminated multidrug resistant (MDR) TB the child was started on ofloxacin, amikacin, ethionamide and clarithromycin with steroids. Subsequently sensitivity to second line drugs was obtained using the drug proportion method which showed M tuberculosis sensitive to para-aminosalicylic acid, ofloxacin and kanamycin but resistant to amikacin, cycloserin and ethionamide. Thus the child was started on ofloxacin, kanamycin, clarithromycin and para-aminosalicylic acid.
OUTCOME AND FOLLOW-UP
The child improved symptomatically, and gained in weight with regression of the hepatosplenomegaly. Ocular movements returned to normal, and proptosis and papilloedema regressed. Abdominal ultrasound at 2 months follow-up showed reduction in the size of splenic abscesses, with the largest lesion measuring 1.3 cm in diameter with central liquefaction. At 9 months follow-up the child continued to remain well, had adequate weight gain, and abdominal ultrasound showed complete resolution of splenic abscesses. Brain MRI showed resolution of tuberculomas with an old infarct in the right parietal region.
DISCUSSION
MDR TB refers to disease caused by M tuberculosis organisms resistant to isoniazid and rifampicin with or without resistance to other drugs.2 The incidence of MDR TB in developing countries, especially India, is probably underestimated and poses a great diagnostic as well as therapeutic challenge.2 In addition, emergence of extensively drug resistant (XDR) TB, defined as resistance to isoniazid and rifampin in addition to any fluoroquinolone and at least one of the following three injectable drugs—capreomycin, kanamycin and amikacin—is further complicating the management issues.3 The index patient was thus a case of XDR TB. Moreover, despite lack of contact with TB, the index patient developed tubercular splenic abscesses and disseminated disease while on standard ATT. We pondered upon the reason for such a unique clinical presentation and primary drug resistance.
We found that with improved understanding of host genetic factors affecting TB, it is likely that many of the apparently immunocompetent children could have an underlying genetic predisposition or certain molecular defects influencing the genesis of conditions leading to atypical and distinct clinical manifestations of TB, and at times disseminated disease in them.4–6 Seneviratne et al reported disseminated M tuberculosis infection due to IFN-γ deficiency which prompted us to perform IFN-γ and IL-12 assay in our case.7 The index patient was found to have absent production of IFN-γ as well as IL-12 which could explain the unusual course of his illness. Authors have also suggested therapeutic use of IFN-γ in disseminated TB.7–9 Defects in IFN-γ axis and Th1 pathway could also be contributory to the emerging problem of XDR TB.
LEARNING POINTS
Although similar problems with immunity to mycobacteria are seen in individuals with defects in IFN-γ receptors, to our knowledge, disseminated XDR Mycobacterium tuberculosis infection due to IFN-γ and IL-12 deficiency has not been previously reported in the paediatric population.
Appropriate epidemiological survey and intensive screening of family members and contacts is warranted in these patients as they may provide important diagnostic clues.
Lack of adequate therapeutic response to standard anti-tubercular treatment should prompt further investigations including mycobacterial cultures, their sensitivity testing and immunological survey, especially in cases with multidrug resistant (MDR) and extensively drug resistant (XDR) TB.
Screening IFN-γ/IL-12 axis of HIV seronegative patients with disseminated TB, XDR TB or progressive TB despite therapy or with unusual manifestations of TB should be considered as it may allow initiation of appropriate life saving treatment.
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication
REFERENCES
- 1.Alcaïs A, Fieschi C, Abel L, et al. Tuberculosis in children and adults: two distinct genetic diseases. J Exp Med 2005; 12: 1617–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amdekar YK. Multidrug resistant tuberculosis. Indian pediatrics 1998; 35: 715–8 [PubMed] [Google Scholar]
- 3.Ravinglione MC, Smith IM. XDR tuberculosis — implications for global public health. N Engl J Med 2007; 356: 656–9 [DOI] [PubMed] [Google Scholar]
- 4.Selvaraj P. Host genetics and tuberculosis susceptibility. Current Science 2004; 86: 115–21 [Google Scholar]
- 5.Lammas DA, Casanova JL, Kumararatne DS. Clinical consequences of defects in the IL-12 dependent interferon-gamma (IFN γ) pathway. Clin Exp Immunol 2000; 121: 417–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newport MJ, Huxley CM, Huston S, et al. A mutation in the interferon-γ-receptor gene and susceptibility to mycobacterial infection. N Engl J Med 1996; 335: 1941–9 [DOI] [PubMed] [Google Scholar]
- 7.Seneviratne Luke, et al. Disseminated Mycobacterium tuberculosis infection due to interferon. Response to replacement therapy. Thorax 2007; 62: 97–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holland SM, Eisenstein EM, Kuhns DB, et al. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N Engl J Med 1994; 330: 1348–55 [DOI] [PubMed] [Google Scholar]
- 9.Reljic R. IFN-γ therapy of tuberculosis and related infections. Journal of Interferon and Cytokine Research 2007; 27: 353–64 [DOI] [PubMed] [Google Scholar]