Abstract
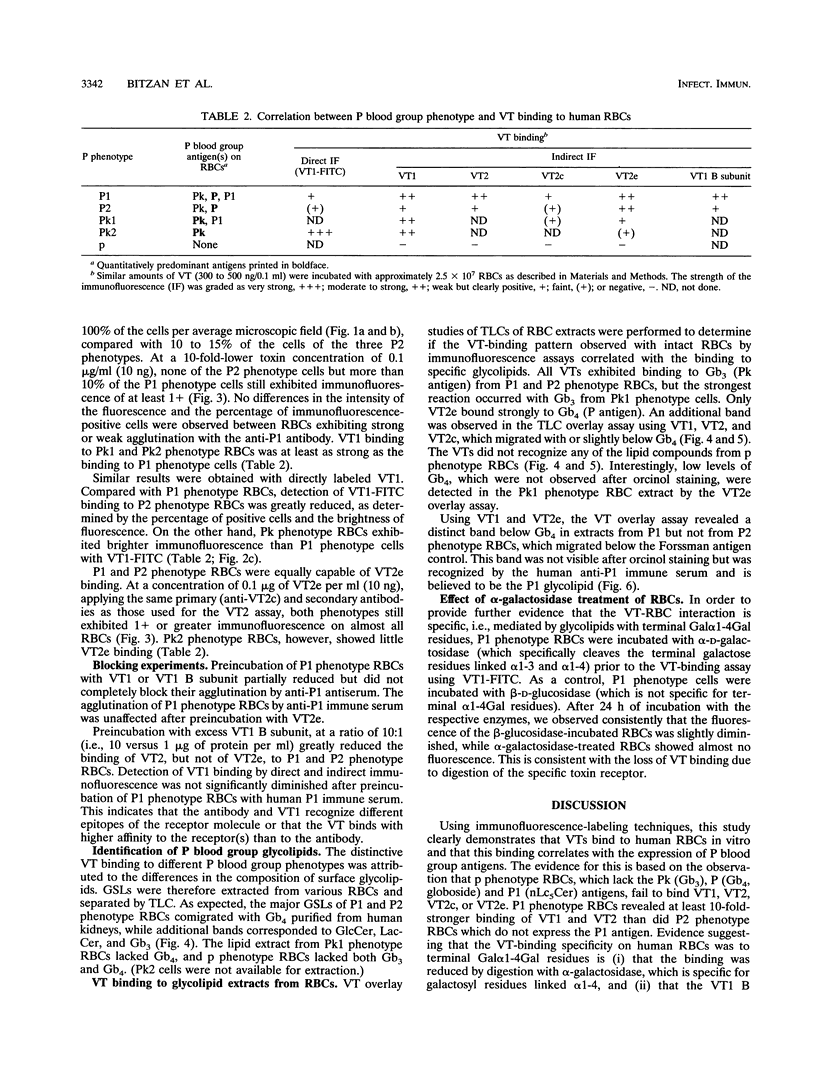
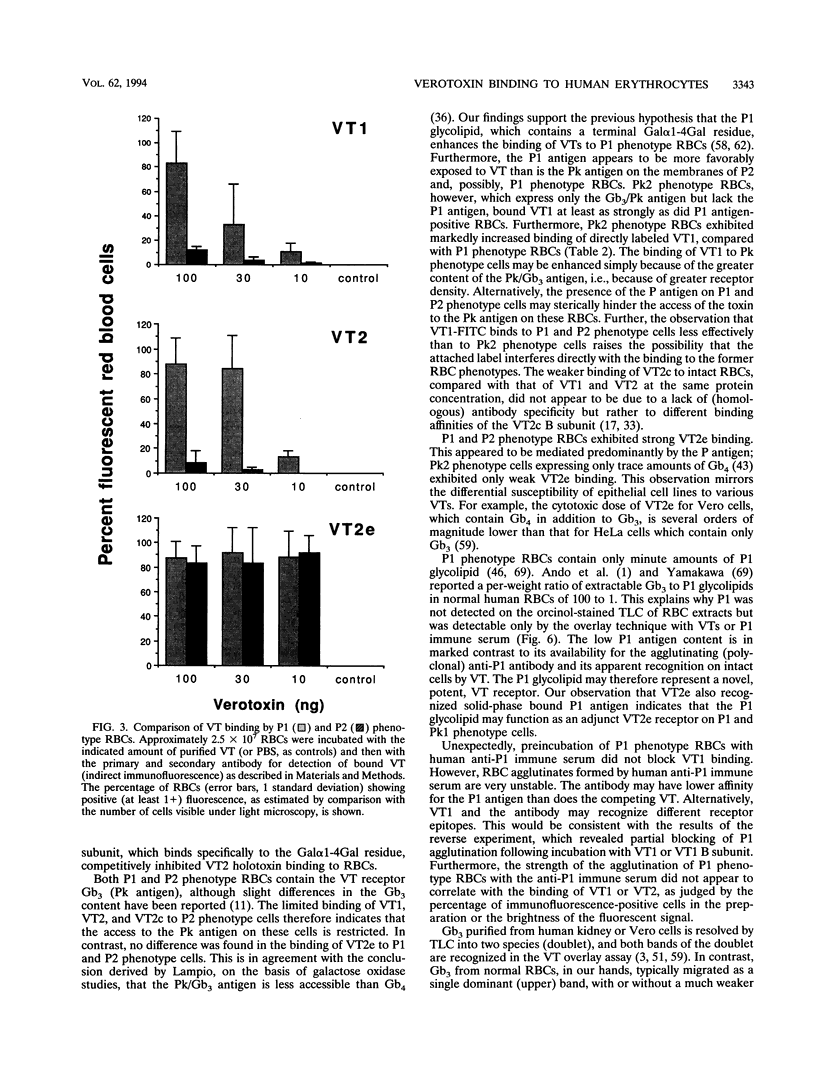
The interaction of verotoxins (VTs) with human erythrocytes (RBCs) in vitro was investigated, with particular reference to the role of P blood group glycolipids that are structurally related to the known VT receptors. RBC binding of purified VT1, VT2, VT2c, and VT2e was detected by direct and indirect immunofluorescence. Glycolipids were extracted from defined RBCs, separated by thin-layer chromatography, and assessed for VT binding in an overlay assay by adding toxin and specific antibodies. All VTs bound to P1 phenotype (Pk, P, and P1 antigens) and P2 phenotype (Pk and P antigens) RBCs but not to p phenotype (lacking the Pk, P, and P1 antigens) RBCs. Binding of VT1 and VT2 was approximately 10-fold greater to P1 and the rare Pk2 (Pk antigen but no P1 or P antigen) phenotype cells than to P2 phenotype RBCs, whereas VT2e bound equally well to P1 and P2 phenotype cells. The VT1 and VT2 immunofluorescence results correlated with the detection of P1 and/or increased amounts of Pk (globotriaosylceramide) antigen; VT2e immunofluorescence correlated with the detection of P (globotetraosylceramide) antigen. The Pk band pattern and VT binding observed in the thin-layer chromatogram of human P1 and P phenotype RBC extracts varied from that of human kidney and Pk1 phenotype (Pk and P1 antigens) RBCs. We conclude that each VT binds to human RBCs in vitro by utilizing specific P blood group glycolipids as receptors. On P1 and P phenotype RBCs, the accessibility of the Pk antigen for VTs appeared to be restricted. The occurrence of VT-RBC binding in natural VT-producing Escherichia coli disease and its relevance for the pathophysiology of hemolytic uremic syndrome remain to be established.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando S., Kon K., Isobe M., Nagai Y., Yamakawa T. Existence of glucosaminyl lactosyl ceramide (amino CTH-I) in human erythrocyte membranes as a possible precursor of blood group-active glycolipids. J Biochem. 1976 Mar;79(3):625–632. doi: 10.1093/oxfordjournals.jbchem.a131106. [DOI] [PubMed] [Google Scholar]
- Bitzan M., Ludwig K., Klemt M., König H., Büren J., Müller-Wiefel D. E. The role of Escherichia coli O 157 infections in the classical (enteropathic) haemolytic uraemic syndrome: results of a Central European, multicentre study. Epidemiol Infect. 1993 Apr;110(2):183–196. doi: 10.1017/s0950268800068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd B., Lingwood C. Verotoxin receptor glycolipid in human renal tissue. Nephron. 1989;51(2):207–210. doi: 10.1159/000185286. [DOI] [PubMed] [Google Scholar]
- Boyd B., Tyrrell G., Maloney M., Gyles C., Brunton J., Lingwood C. Alteration of the glycolipid binding specificity of the pig edema toxin from globotetraosyl to globotriaosyl ceramide alters in vivo tissue targetting and results in a verotoxin 1-like disease in pigs. J Exp Med. 1993 Jun 1;177(6):1745–1753. doi: 10.1084/jem.177.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain M. C. The haemolytic-uraemic syndrome. Semin Hematol. 1969 Apr;6(2):162–180. [PubMed] [Google Scholar]
- Bull B. S., Rubenberg M. L., Dacie J. V., Brain M. C. Red-blood-cell fragmentation in microangiopathic haemolytic anaemia: in-vitro studies. Lancet. 1967 Nov 25;2(7526):1123–1125. doi: 10.1016/s0140-6736(67)90623-x. [DOI] [PubMed] [Google Scholar]
- Byrnes J. J., Moake J. L. Thrombotic thrombocytopenic purpura and the haemolytic-uraemic syndrome: evolving concepts of pathogenesis and therapy. Clin Haematol. 1986 May;15(2):413–442. [PubMed] [Google Scholar]
- DeGrandis S., Law H., Brunton J., Gyles C., Lingwood C. A. Globotetraosylceramide is recognized by the pig edema disease toxin. J Biol Chem. 1989 Jul 25;264(21):12520–12525. [PubMed] [Google Scholar]
- Downes F. P., Barrett T. J., Green J. H., Aloisio C. H., Spika J. S., Strockbine N. A., Wachsmuth I. K. Affinity purification and characterization of Shiga-like toxin II and production of toxin-specific monoclonal antibodies. Infect Immun. 1988 Aug;56(8):1926–1933. doi: 10.1128/iai.56.8.1926-1933.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher K. S., Bremer E. G., Schwarting G. A. P blood group regulation of glycosphingolipid levels in human erythrocytes. J Biol Chem. 1979 Nov 25;254(22):11196–11198. [PubMed] [Google Scholar]
- Fong J. S., de Chadarevian J. P., Kaplan B. S. Hemolytic-uremic syndrome. Current concepts and management. Pediatr Clin North Am. 1982 Aug;29(4):835–856. doi: 10.1016/s0031-3955(16)34216-x. [DOI] [PubMed] [Google Scholar]
- GASSER C., GAUTIER E., STECK A., SIEBENMANN R. E., OECHSLIN R. Hämolytisch-urämische Syndrome: bilaterale Nierenrindennekrosen bei akuten erworbenen hämolytischen Anämien. Schweiz Med Wochenschr. 1955 Sep 20;85(38-39):905–909. [PubMed] [Google Scholar]
- GIANANTONIO C., VITACCO M., MENDILAHARZU F., RUTTY A., MENDILAHARZU J. THE HEMOLYTIC-UREMIC SYNDROME. J Pediatr. 1964 Apr;64:478–491. doi: 10.1016/s0022-3476(64)80337-1. [DOI] [PubMed] [Google Scholar]
- Habib R., Mathieu H., Royer P. Le syndrome hémolytique et urémique de l'enfant,. Aspects cliniques et anatomiques dans 27 observations. Nephron. 1967;4(3):139–172. doi: 10.1159/000179580. [DOI] [PubMed] [Google Scholar]
- Head S. C., Karmali M. A., Lingwood C. A. Preparation of VT1 and VT2 hybrid toxins from their purified dissociated subunits. Evidence for B subunit modulation of a subunit function. J Biol Chem. 1991 Feb 25;266(6):3617–3621. [PubMed] [Google Scholar]
- Head S. C., Karmali M. A., Roscoe M. E., Petric M., Strockbine N. A., Wachsmuth I. K. Serological differences between verocytotoxin 2 and shiga-like toxin II. Lancet. 1988 Sep 24;2(8613):751–751. doi: 10.1016/s0140-6736(88)90228-0. [DOI] [PubMed] [Google Scholar]
- Head S., Ramotar K., Lingwood C. Modification of the glycolipid-binding specificity of vero cytotoxin by polymyxin B and other cyclic amphipathic peptides. Infect Immun. 1990 Jun;58(6):1532–1537. doi: 10.1128/iai.58.6.1532-1537.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hii J. H., Gyles C., Morooka T., Karmali M. A., Clarke R., De Grandis S., Brunton J. L. Development of verotoxin 2- and verotoxin 2 variant (VT2v)-specific oligonucleotide probes on the basis of the nucleotide sequence of the B cistron of VT2v from Escherichia coli E32511 and B2F1. J Clin Microbiol. 1991 Dec;29(12):2704–2709. doi: 10.1128/jcm.29.12.2704-2709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A., de Grandis S., Friesen J., Karmali M., Petric M., Congi R., Brunton J. L. Cloning and expression of the genes specifying Shiga-like toxin production in Escherichia coli H19. J Bacteriol. 1986 May;166(2):375–379. doi: 10.1128/jb.166.2.375-379.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Terai A., Kurazono H., Takeda Y., Nishibuchi M. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb Pathog. 1990 Jan;8(1):47–60. doi: 10.1016/0882-4010(90)90007-d. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Kaplan B. S., Cleary T. G., Obrig T. G. Recent advances in understanding the pathogenesis of the hemolytic uremic syndromes. Pediatr Nephrol. 1990 May;4(3):276–283. doi: 10.1007/BF00857676. [DOI] [PubMed] [Google Scholar]
- Karmali M. A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989 Jan;2(1):15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., Petric M., Lim C., Fleming P. C., Arbus G. S., Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985 May;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- Kaye S. A., Louise C. B., Boyd B., Lingwood C. A., Obrig T. G. Shiga toxin-associated hemolytic uremic syndrome: interleukin-1 beta enhancement of Shiga toxin cytotoxicity toward human vascular endothelial cells in vitro. Infect Immun. 1993 Sep;61(9):3886–3891. doi: 10.1128/iai.61.9.3886-3891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarash A., Boyd B., Lingwood C. A. Glycosphingolipid receptor function is modified by fatty acid content. Verotoxin 1 and verotoxin 2c preferentially recognize different globotriaosyl ceramide fatty acid homologues. J Biol Chem. 1994 Apr 15;269(15):11138–11146. [PubMed] [Google Scholar]
- Kleanthous H., Smith H. R., Scotland S. M., Gross R. J., Rowe B., Taylor C. M., Milford D. V. Haemolytic uraemic syndromes in the British Isles, 1985-8: association with verocytotoxin producing Escherichia coli. Part 2: Microbiological aspects. Arch Dis Child. 1990 Jul;65(7):722–727. doi: 10.1136/adc.65.7.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp W., Dörken B., Rieber P., Schmidt R. E., Stein H., von dem Borne A. E. CD antigens 1989. Int J Cancer. 1989 Jul 15;44(1):190–191. doi: 10.1002/ijc.2910440135. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Brown J. E., Strömberg N., Westling-Ryd M., Schultz J. E., Karlsson K. A. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987 Feb 5;262(4):1779–1785. [PubMed] [Google Scholar]
- Linggood M. A., Thompson J. M. Verotoxin production among porcine strains of Escherichia coli and its association with oedema disease. J Med Microbiol. 1987 Dec;24(4):359–362. doi: 10.1099/00222615-24-4-359. [DOI] [PubMed] [Google Scholar]
- Lingwood C. A., Law H., Richardson S., Petric M., Brunton J. L., De Grandis S., Karmali M. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem. 1987 Jun 25;262(18):8834–8839. [PubMed] [Google Scholar]
- Lingwood C. A. Verotoxins and their glycolipid receptors. Adv Lipid Res. 1993;25:189–211. [PubMed] [Google Scholar]
- MacLeod D. L., Gyles C. L., Wilcock B. P. Reproduction of edema disease of swine with purified Shiga-like toxin-II variant. Vet Pathol. 1991 Jan;28(1):66–73. doi: 10.1177/030098589102800109. [DOI] [PubMed] [Google Scholar]
- Marcus D. M., Kundu S. K., Suzuki A. The P blood group system: recent progress in immunochemistry and genetics. Semin Hematol. 1981 Jan;18(1):63–71. [PubMed] [Google Scholar]
- Marcus D. M., Naiki M., Kundu S. K. Abnormalities in the glycosphingolipid content of human Pk and p erythrocytes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3263–3267. doi: 10.1073/pnas.73.9.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Karch H., Hacker J., Bocklage H., Heesemann J. Cloning and sequencing of a Shiga-like toxin II-related gene from Escherichia coli O157:H7 strain 7279. Zentralbl Bakteriol. 1992 Jan;276(2):176–188. doi: 10.1016/s0934-8840(11)80004-6. [DOI] [PubMed] [Google Scholar]
- Milford D. V., Taylor C. M., Rose P. E., Roy T. C., Rowe B. Immunologic therapy for hemolytic-uremic syndrome. J Pediatr. 1989 Sep;115(3):502–504. doi: 10.1016/s0022-3476(89)80872-8. [DOI] [PubMed] [Google Scholar]
- Naiki M., Marcus D. M. An immunochemical study of the human blood group P1, P, and PK glycosphingolipid antigens. Biochemistry. 1975 Nov 4;14(22):4837–4841. doi: 10.1021/bi00693a010. [DOI] [PubMed] [Google Scholar]
- Newburg D. S., Chaturvedi P., Lopez E. L., Devoto S., Fayad A., Cleary T. G. Susceptibility to hemolytic-uremic syndrome relates to erythrocyte glycosphingolipid patterns. J Infect Dis. 1993 Aug;168(2):476–479. doi: 10.1093/infdis/168.2.476. [DOI] [PubMed] [Google Scholar]
- Obrig T. G., Louise C. B., Lingwood C. A., Boyd B., Barley-Maloney L., Daniel T. O. Endothelial heterogeneity in Shiga toxin receptors and responses. J Biol Chem. 1993 Jul 25;268(21):15484–15488. [PubMed] [Google Scholar]
- Oku Y., Yutsudo T., Hirayama T., O'Brien A. D., Takeda Y. Purification and some properties of a Vero toxin from a human strain of Escherichia coli that is immunologically related to Shiga-like toxin II (VT2). Microb Pathog. 1989 Feb;6(2):113–122. doi: 10.1016/0882-4010(89)90014-4. [DOI] [PubMed] [Google Scholar]
- Pellizzari A., Pang H., Lingwood C. A. Binding of verocytotoxin 1 to its receptor is influenced by differences in receptor fatty acid content. Biochemistry. 1992 Feb 11;31(5):1363–1370. doi: 10.1021/bi00120a011. [DOI] [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Ramotar K., Boyd B., Tyrrell G., Gariepy J., Lingwood C., Brunton J. Characterization of Shiga-like toxin I B subunit purified from overproducing clones of the SLT-I B cistron. Biochem J. 1990 Dec 15;272(3):805–811. doi: 10.1042/bj2720805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. E., Karmali M. A., Becker L. E., Smith C. R. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum Pathol. 1988 Sep;19(9):1102–1108. doi: 10.1016/s0046-8177(88)80093-5. [DOI] [PubMed] [Google Scholar]
- Richardson S. E., Rotman T. A., Jay V., Smith C. R., Becker L. E., Petric M., Olivieri N. F., Karmali M. A. Experimental verocytotoxemia in rabbits. Infect Immun. 1992 Oct;60(10):4154–4167. doi: 10.1128/iai.60.10.4154-4167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Remis R. S., Helgerson S. D., McGee H. B., Wells J. G., Davis B. R., Hebert R. J., Olcott E. S., Johnson L. M., Hargrett N. T. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983 Mar 24;308(12):681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- Rose P. E., Clark A. J. Haematology of the haemolytic uraemic syndrome. Blood Rev. 1989 Jun;3(2):136–140. doi: 10.1016/0268-960x(89)90009-x. [DOI] [PubMed] [Google Scholar]
- Samuel J. E., Perera L. P., Ward S., O'Brien A. D., Ginsburg V., Krivan H. C. Comparison of the glycolipid receptor specificities of Shiga-like toxin type II and Shiga-like toxin type II variants. Infect Immun. 1990 Mar;58(3):611–618. doi: 10.1128/iai.58.3.611-618.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C. K., McKee M. L., O'Brien A. D. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H- strain E32511. Infect Immun. 1991 Mar;59(3):1065–1073. doi: 10.1128/iai.59.3.1065-1073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr P. I., Neill M. A., Clausen C. R., Newland J. W., Neill R. J., Moseley S. L. Genotypic variation in pathogenic Escherichia coli O157:H7 isolated from patients in Washington, 1984-1987. J Infect Dis. 1989 Feb;159(2):344–347. doi: 10.1093/infdis/159.2.344. [DOI] [PubMed] [Google Scholar]
- Taylor C. M., Milford D. V., Rose P. E., Roy T. C., Rowe B. The expression of blood group P1 in post-enteropathic haemolytic uraemic syndrome. Pediatr Nephrol. 1990 Jan;4(1):59–61. doi: 10.1007/BF00858441. [DOI] [PubMed] [Google Scholar]
- Tyrrell G. J., Ramotar K., Toye B., Boyd B., Lingwood C. A., Brunton J. L. Alteration of the carbohydrate binding specificity of verotoxins from Gal alpha 1-4Gal to GalNAc beta 1-3Gal alpha 1-4Gal and vice versa by site-directed mutagenesis of the binding subunit. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):524–528. doi: 10.1073/pnas.89.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman M. D., McCluer R. H. Quantitative analysis of plasma neutral glycosphingolipids by high performance liquid chromatography of their perbenzoyl derivatives. J Lipid Res. 1977 May;18(3):371–378. [PubMed] [Google Scholar]
- Weinstein D. L., Jackson M. P., Samuel J. E., Holmes R. K., O'Brien A. D. Cloning and sequencing of a Shiga-like toxin type II variant from Escherichia coli strain responsible for edema disease of swine. J Bacteriol. 1988 Sep;170(9):4223–4230. doi: 10.1128/jb.170.9.4223-4230.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kar N. C., Monnens L. A., Karmali M. A., van Hinsbergh V. W. Tumor necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the hemolytic uremic syndrome. Blood. 1992 Dec 1;80(11):2755–2764. [PubMed] [Google Scholar]








