Abstract
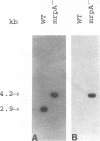
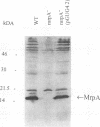
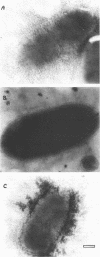
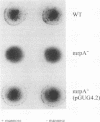
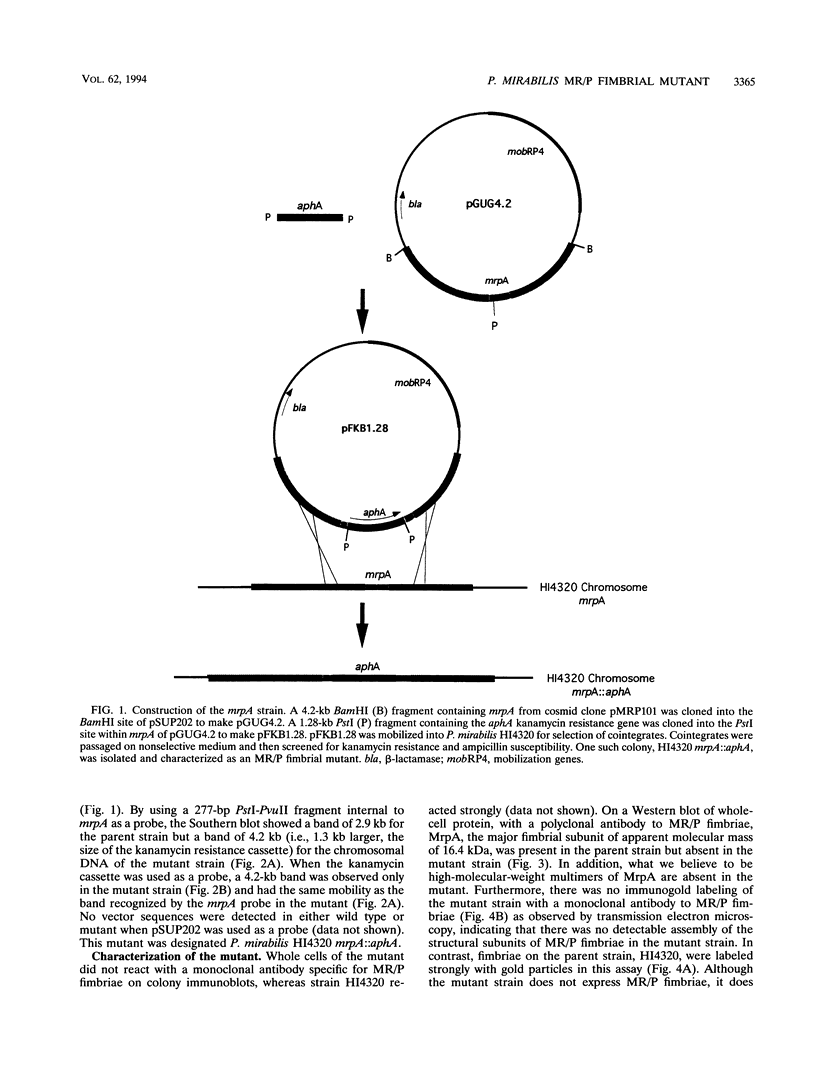
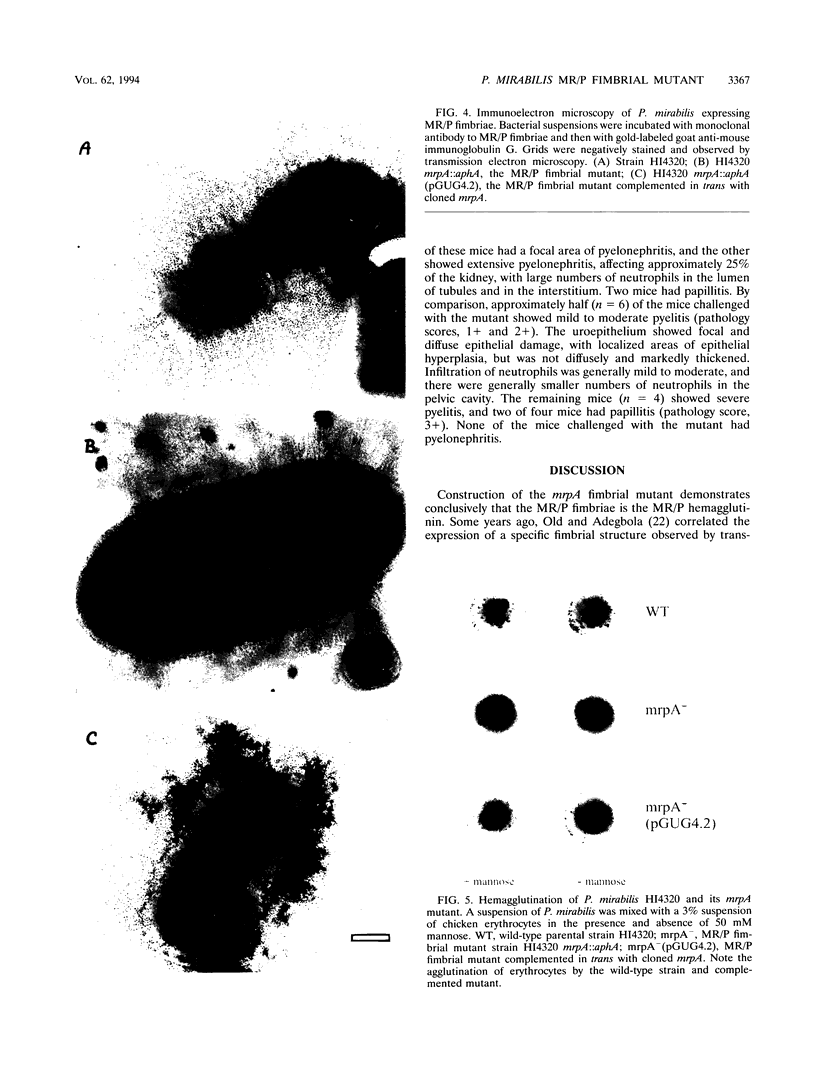
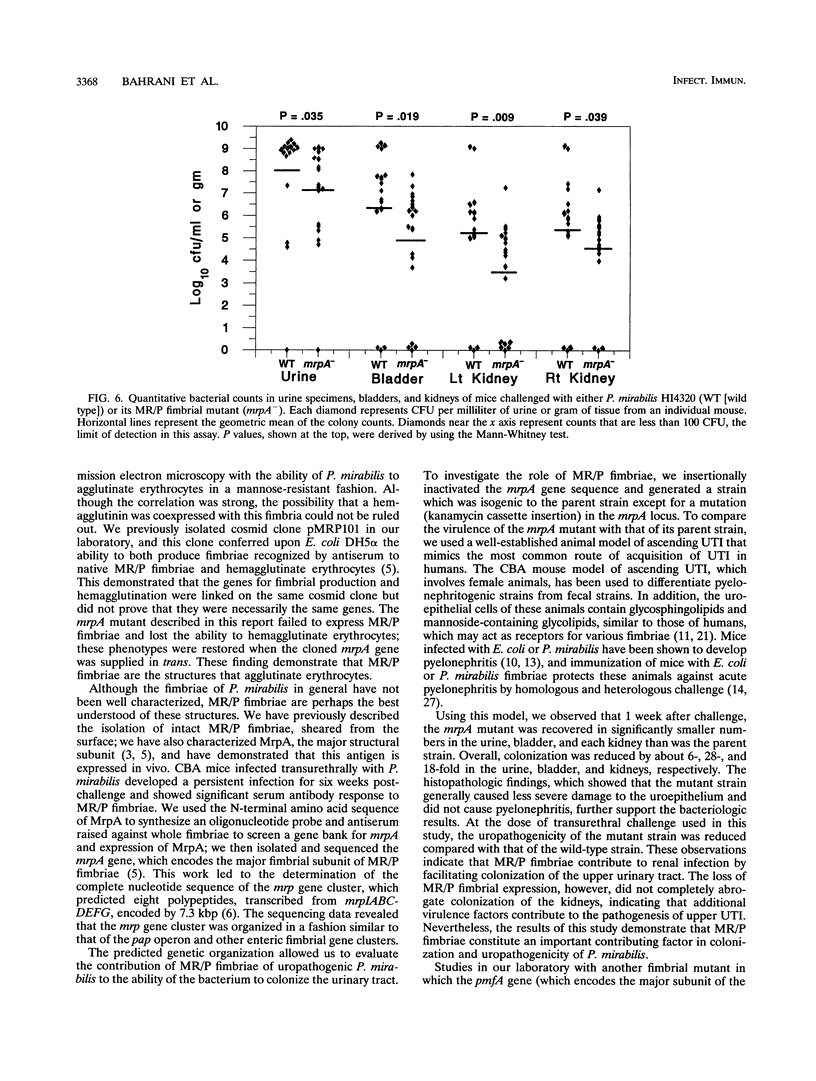
Proteus mirabilis, a cause of acute pyelonephritis, produces at least four types of fimbriae, including MR/P (mannose-resistant/Proteus-like) fimbriae. To investigate the contribution of MR/P fimbriae to colonization of the urinary tract, we constructed an MR/P fimbrial mutant by allelic exchange. A 4.2-kb BamHI fragment carrying the mrpA gene was subcloned into a mobilizable plasmid, pSUP202. A 1.3-kb Kanr cassette was inserted into the mrpA open reading frame, and the construct was transferred to the parent P. mirabilis strain by conjugation. Following passage on nonselective medium, 1 of 500 transconjugants screened was found to have undergone allelic exchange as demonstrated by Southern blot. Colony immunoblot, Western immunoblot, and immunogold labeling with a monoclonal antibody to MR/P fimbriae revealed that MrpA was not expressed. Complementation with cloned mrpA restored MR/P expression as shown by hemagglutination, Western blot, and immunogold electron microscopy. To assess virulence, we challenged 40 CBA mice transurethrally with 10(7) CFU of wild-type or mutant strains. After 1 week, geometric means of log10 CFU per milliliter of urine or per gram of bladder or kidney for the wild-type and mutant strains were as follows: urine, 7.79 (wild type) versus 7.02 (mutant) (P = 0.035); bladder, 6.22 versus 4.78 (P = 0.019); left kidney, 5.02 versus 3.31 (P = 0.009); and right kidney, 5.28 versus 4.46 (P = 0.039). Mice challenged with the wild-type strain showed significantly more severe renal damage than did mice challenged with the MR/P-negative mutant (P = 0.007). We conclude that MR/P fimbriae contribute significantly to colonization of the urinary tract and increase the risk of development of acute pyelonephritis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adegbola R. A., Old D. C., Senior B. W. The adhesins and fimbriae of Proteus mirabilis strains associated with high and low affinity for the urinary tract. J Med Microbiol. 1983 Nov;16(4):427–431. doi: 10.1099/00222615-16-4-427. [DOI] [PubMed] [Google Scholar]
- Bahrani F. K., Cook S., Hull R. A., Massad G., Mobley H. L. Proteus mirabilis fimbriae: N-terminal amino acid sequence of a major fimbrial subunit and nucleotide sequences of the genes from two strains. Infect Immun. 1993 Mar;61(3):884–891. doi: 10.1128/iai.61.3.884-891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrani F. K., Johnson D. E., Robbins D., Mobley H. L. Proteus mirabilis flagella and MR/P fimbriae: isolation, purification, N-terminal analysis, and serum antibody response following experimental urinary tract infection. Infect Immun. 1991 Oct;59(10):3574–3580. doi: 10.1128/iai.59.10.3574-3580.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrani F. K., Mobley H. L. Proteus mirabilis MR/P fimbriae: molecular cloning, expression, and nucleotide sequence of the major fimbrial subunit gene. J Bacteriol. 1993 Jan;175(2):457–464. doi: 10.1128/jb.175.2.457-464.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrani F. K., Mobley H. L. Proteus mirabilis MR/P fimbrial operon: genetic organization, nucleotide sequence, and conditions for expression. J Bacteriol. 1994 Jun;176(11):3412–3419. doi: 10.1128/jb.176.11.3412-3419.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Larsson P., Lomberg H. Attachment of Proteus mirabilis to human urinary sediment epithelial cells in vitro is different from that of Escherichia coli. Infect Immun. 1980 Mar;27(3):804–807. doi: 10.1128/iai.27.3.804-807.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Taylor G. M. An immunocolloid method for the electron microscope. Immunochemistry. 1971 Nov;8(11):1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Hagberg L., Engberg I., Freter R., Lam J., Olling S., Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983 Apr;40(1):273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. E., Lockatell C. V., Hall-Craigs M., Mobley H. L., Warren J. W. Uropathogenicity in rats and mice of Providencia stuartii from long-term catheterized patients. J Urol. 1987 Sep;138(3):632–635. doi: 10.1016/s0022-5347(17)43287-3. [DOI] [PubMed] [Google Scholar]
- Jones B. D., Lockatell C. V., Johnson D. E., Warren J. W., Mobley H. L. Construction of a urease-negative mutant of Proteus mirabilis: analysis of virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1990 Apr;58(4):1120–1123. doi: 10.1128/iai.58.4.1120-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legnani-Fajardo C., Zunino P., Algorta G., Laborde H. F. Antigenic and immunogenic activity of flagella and fimbriae preparations from uropathogenic Proteus mirabilis. Can J Microbiol. 1991 Apr;37(4):325–328. doi: 10.1139/m91-052. [DOI] [PubMed] [Google Scholar]
- Massad G., Lockatell C. V., Johnson D. E., Mobley H. L. Proteus mirabilis fimbriae: construction of an isogenic pmfA mutant and analysis of virulence in a CBA mouse model of ascending urinary tract infection. Infect Immun. 1994 Feb;62(2):536–542. doi: 10.1128/iai.62.2.536-542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R. Hemagglutinin, urease, and hemolysin production by Proteus mirabilis from clinical sources. J Infect Dis. 1990 Mar;161(3):525–530. doi: 10.1093/infdis/161.3.525. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R., Swihart K. G., Welch R. A. Cytotoxicity of the HpmA hemolysin and urease of Proteus mirabilis and Proteus vulgaris against cultured human renal proximal tubular epithelial cells. Infect Immun. 1991 Jun;59(6):2036–2042. doi: 10.1128/iai.59.6.2036-2042.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R., Tenney J. H., Warren J. W. Adherence to uroepithelial cells of Providencia stuartii isolated from the catheterized urinary tract. J Gen Microbiol. 1986 Oct;132(10):2863–2872. doi: 10.1099/00221287-132-10-2863. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Warren J. W. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987 Nov;25(11):2216–2217. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Falkow S., Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985 Feb;75(2):347–360. doi: 10.1172/JCI111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Adegbola R. A. Haemagglutinins and fimbriae of Morganella, Proteus and Providencia. J Med Microbiol. 1982 Nov;15(4):551–564. doi: 10.1099/00222615-15-4-551. [DOI] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Investigation of the haemolytic activity of Proteus mirabilis strains. Antonie Van Leeuwenhoek. 1983 Apr;49(1):1–11. doi: 10.1007/BF00457874. [DOI] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Vero cell invasiveness of Proteus mirabilis. Infect Immun. 1984 Mar;43(3):1068–1071. doi: 10.1128/iai.43.3.1068-1071.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareneva T., Holthöfer H., Korhonen T. K. Tissue-binding affinity of Proteus mirabilis fimbriae in the human urinary tract. Infect Immun. 1990 Oct;58(10):3330–3336. doi: 10.1128/iai.58.10.3330-3336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. A., O'Hanley P., Lark D., Schoolnik G. K. Synthetic peptides corresponding to protective epitopes of Escherichia coli digalactoside-binding pilin prevent infection in a murine pyelonephritis model. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1247–1251. doi: 10.1073/pnas.85.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B. W., Albrechtsen M., Kerr M. A. Proteus mirabilis strains of diverse type have IgA protease activity. J Med Microbiol. 1987 Sep;24(2):175–180. doi: 10.1099/00222615-24-2-175. [DOI] [PubMed] [Google Scholar]
- Setia U., Serventi I., Lorenz P. Bacteremia in a long-term care facility. Spectrum and mortality. Arch Intern Med. 1984 Aug;144(8):1633–1635. [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Influence of pili on the virulence of Proteus mirabilis in experimental hematogenous pyelonephritis. J Infect Dis. 1978 Nov;138(5):664–667. doi: 10.1093/infdis/138.5.664. [DOI] [PubMed] [Google Scholar]
- Swihart K. G., Welch R. A. Cytotoxic activity of the Proteus hemolysin HpmA. Infect Immun. 1990 Jun;58(6):1861–1869. doi: 10.1128/iai.58.6.1861-1869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982 Dec;146(6):719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- Wray S. K., Hull S. I., Cook R. G., Barrish J., Hull R. A. Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis. Infect Immun. 1986 Oct;54(1):43–49. doi: 10.1128/iai.54.1.43-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]