Abstract
Borrelia spp. are pathogens responsible for worldwide tickborne relapsing fever (TBRF). In West Africa, TBRF is due to a single species, Borrelia crocidurae, transmitted by the soft-body tick Ornithodoros sonrai. We report a case of B crocidurae infection in a French tourist in Senegal, diagnosed by molecular biology using 16S rDNA, flaB, and the 16S–23S intergenic spacer. We found six imported cases reported in travellers (since 1999). We review here clinical and molecular aspects and pathophysiology, and discuss diagnostic methods and therapeutic regimens. In the coming years, this emerging disease will be of concern to more and more travellers returning from disease-endemic regions. Thus, physicians must be aware of its presentation and diagnosis, since the spontaneous outcome can be severe, and a simple treatment is effective.
BACKGROUND
In the coming years, this emerging disease will be of concern to more and more travellers returning from Africa because of increasing international transportation and rising incidence in West Africa. Thus, physicians must be aware of its presentation and diagnosis, since the untreated course can be severe, while a simple treatment is effective.
CASE PRESENTATION
A 48-year-old French woman was admitted to the Infectious Diseases and Tropical Medicine ward in North Hospital in Marseille, France, for a flu-like syndrome with high-grade fever. She had returned from Senegal for 4 days, where she had travelled for 1 week to Dakar and Saly (Mbour province, 80 km south of Dakar). She presented with high-grade fever (40°C), chills, headache, muscle and joint pain and unproductive cough. Clinical examination showed conjunctivitis.
INVESTIGATIONS
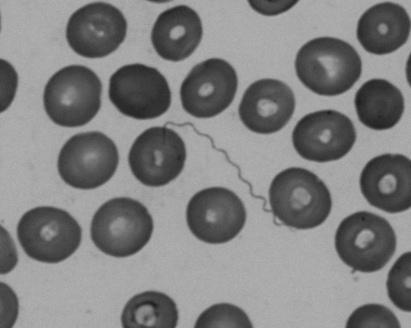
Analyses revealed haemoglobin 10.9 g/dl, thrombocytopenia (55×109/l) and elevated C-reactive protein (302 mg/l). Giemsa-stained thick blood smears showed no malaria parasites but helical bacteria suggestive of spirochetes (fig 1). Serology for Borrelia burgdorferi was negative. Partial amplification of the 16S rRNA gene from blood using 536 F/rp2 PCR primers yielded a sequence exhibiting 99% similarity with that of Borrelia crocidurae (GenBank accession number U42302) over 995 base-pairs; sequencing of flaB yielded 98% similarity over 363 base-pairs; sequencing the 16S–23S rRNA genes intergenic spacer revealed 580/583 (99%) sequence similarity with that of B crocidurae (GenBank accession number DQ000287) and 553/588 (94%) sequence similarity with that of Borrelia duttonii (GenBank accession number DQ000282).
Figure 1.
Giemsa-stained thick blood smear showing spirochetes.
TREATMENT
The patient was treated with doxycycline 100 mg twice a day for 10 days.
OUTCOME AND FOLLOW-UP
Fever disappeared on the third day. C-reactive protein decreased dramatically from 200 to 6 mg/l in 5 days. No adverse reactions were observed, and the patient achieved full recovery. One month later, the patient was free of symptoms.
DISCUSSION
Tickborne relapsing fever is caused by at least 15 distinct Borrelia species throughout the world.1 In West Africa, endemic TBRF is a reemerging disease caused by a spirochete, B crocidurae, although B duttonii and Borrelia recurrentis are also found in Senegal.2 Although the first human case of B crocidurae infection was reported in 1928 in Dakar, Senegal,3 the spread of the disease in West Africa has been highlighted only recently4 and is now recognised as an emerging infection.5 Reservoir hosts are usually wild rodents. B crocidurae is phylogenetically associated with Old World relapsing fever, including B duttonii (Central and East Africa tickborne relapsing fever) and B recurrentis (agent of louseborne relapsing fever (LBRF)).6 Molecular identification of B crocidurae from vector Ornithodoros sonrai or humans (similar geographical repartition) has been reported in Morocco, Mauritania, Mali, Senegal, Gambia and Togo.2,6–11 Among other endemic regions cited in the literature (Egypt, Libya, Turkey, Iran and Kenya),1,12–14 no molecular studies have been conducted to ascertain the homology between these strains and B crocidurae. In Senegal, Vial et al found an average incidence of 11 person years from 1990 to 2003 with a maximum in March (dry season); this extremely high incidence is similar to the incidence reported for malaria.8 The spread of the vector and the disease in West Africa is related to the spread of drought, where the average rainfall is less than 750 mm.4 Since 1999, six imported cases have been reported in travellers from Senegal,9,10,15 Gambia,10 Mali and Mauritania.7 Clinical presentation was a flu-like syndrome,9,10,15 febrile diarrhoea and fever with premature birth7 and meningitis.10 TBRF is characterised by recurrent episodes of fever that accompany spirochetemia. Clinical manifestations appear suddenly 3–12 days after exposure with high temperature, chills, headache, muscle and joint pain, non-productive cough and iritis. Physical examination can show conjunctivitis, splenomegaly and enlarged liver.9 Meningo-encephalitis, myocarditis accompanied by arrhythmia, and liver dysfunction are rare fatal complications. Each crisis lasts 3–6 days, followed by afebrile periods of increasing length from 7 to 10 days. Most often, laboratory findings show no abnormalities or only elevated erythrocyte sedimentation rate,7 but elevated C-reactive protein, elevated lactate dehydrogenase, moderate anaemia, thrombocytopenia and cerebrospinal fluid pleiocytosis have been reported.7,10 Several infections can occur in one person, indicating that there is no acquired immunity.8 Borrelia spp. evade specific immunity by multiphasic antigenic variation, a strategy supported by the variable membrane proteins (Vmps). The expressed Vmp genes change by DNA rearrangement every 104 cell generation.16 In addition, B crocidurae forms aggregates with erythrocytes (erythrocyte rosetting) resulting in a delayed immune response, microemboli and petechial haemorrhages.16 Human IgM antibodies are able to eliminate B crocidurae in blood and most tissues, but they are not successful in clearing borreliae from the brain, cerebrospinal fluid and eye, so spirochetes may persist for years in the central nervous system (CNS).17 Residual brain infection and multiphasic antigenic variation could explain fever relapses. The gold-standard diagnosis of TBRF is direct visualisation of borreliae in Giemsa-stained thick blood smear. Quantitative buffy-coat (QBC) analysis might increase the detection sensitivity.15 Ideally, blood samples should be obtained during febrile episodes, but a positive blood smear in non-febrile patient has been reported.7 In vitro or animal cultivation is possible but is now largely obsolete owing to PCR. To date, no reliable serology exists for B crocidurae infection. B burgdorferi and B recurrentis IgM ELISA have been used, since it is thought that an appreciable cross-reacting antigen homology exists between these species and B crocidurae. Recently, glycerophosphodiester phosphodiesterase (glpQ) has been proposed as a more specific recombinant antigen for serology, distinguishing TBRF from Lyme-related Borrelia spp., but this test is not species-specific; it cannot date the infection and is not commercially available.18 Detection of Borrelia DNA by PCR amplification from the blood is highly sensitive and specific. Identification can be achieved by sequencing the 16S rRNA gene (689 bp), the flagellin gene (flaB) (346–350 bp),19 glpQ20 or an intergenic spacer region gene (IGS, between 16S and 23S rRNA genes)21 as in the case reported herein.
The recommended treatment is doxycycline 100 mg twice daily or erythromycin 500 mg every 6 h PO. Ceftriaxone 2 g daily or penicillin G intravenously is indicated if CNS involvement is suspected. Soon after the initiation of antibiotic therapy, a Jarisch–Herxheimer reaction could occur and be severe (fatal cases reported) and must be anticipated. Mortality, from 2 to 5% without treatment, is less than 2% with appropriate antibiotics.10 Prevention is possible by filling animal burrows or using repellents and insecticides. In a double-bind, placebo-controlled trial, postexposure treatment with doxycycline (200 mg the first day and then 100 mg per day for 4 days) was 100% effective and well tolerated for Borrelia persica.22 Doxycycline prophylaxis did not cause a Jarisch–Herxeimer reaction in any of the subjects treated pre-emptively, while eight of the 10 patients treated after the onset of symptoms developed such a reaction. This result could be extended to West Africa TBRF.
LEARNING POINTS
An increase in intercontinental transportation has led to frequent imported TBRF cases, so it must now be considered in the diagnosis of every febrile patient returning from Africa. Since, in the coming years, this emerging disease will affect increasing numbers of travellers, physicians must be aware of its presentation and diagnosis, since the spontaneous outcome can be severe, and a simple treatment is effective. Diagnosis relies upon careful examination of stained thick blood smears, after buffy coat concentration if possible. When available, molecular methods are highly effective in detecting and identifying bacterial species. Doxycycline is the recommended treatment, although it can cause severe Jarisch–Herxeimer reaction. Finally, postexposure treatment is effective and well tolerated, and must be discussed in endemic zones.
Acknowledgments
We thank SJ Dumler for his help in English reviewing of the manuscript.
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication
REFERENCES
- 1.Rebaudet S, Parola P. Epidemiology of relapsing fever borreliosis in Europe. FEMS Immunol Med Microbiol 2006; 48: 11–15 [DOI] [PubMed] [Google Scholar]
- 2.Brahim H, Perrier-Gros-Claude JD, Postic D, et al. Identifying relapsing fever Borrelia, Senegal. Emerg Infect Dis 2005; 11: 474–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathis C. Identité, à Dakar, du spirochète des rats, du spirochète de la musaraigne, et du spirochète récurrent humain. Bull Soc Pathol Exot 1928; 21: 472–85 [Google Scholar]
- 4.Trape JF, Godeluck B, Diatta G, et al. The spread of tick-borne borreliosis in West Africa and its relationship to sub-Saharan drought. Am J Trop Med Hyg 1996; 54: 289–93 [DOI] [PubMed] [Google Scholar]
- 5.Cutler SJ. Possibilities for relapsing fever reemergence. Emerg Infect Dis 2006; 12: 369–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ras NM, Lascola B, Postic D, et al. Phylogenesis of relapsing fever Borrelia spp. Int J Syst Bacteriol 1996; 46: 859–65 [DOI] [PubMed] [Google Scholar]
- 7.Wyplosz B, Mihaila-Amrouche L, Baixench MT, et al. Imported tickborne relapsing fever, France. Emerg Infect Dis 2005; 11: 1801–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vial L, Diatta G, Tall A, et al. Incidence of tick-borne relapsing fever in west Africa: longitudinal study. Lancet 2006; 368: 37–43 [DOI] [PubMed] [Google Scholar]
- 9.Tordini G, Giaccherini R, Corbisiero R, et al. Relapsing fever in a traveller from Senegal: determination of Borrelia species using molecular methods. Trans R Soc Trop Med Hyg 2006; 100: 992–4 [DOI] [PubMed] [Google Scholar]
- 10.van Dam AP, van GT, Wetsteyn JC, et al. Tick-borne relapsing fever imported from West Africa: diagnosis by quantitative buffy coat analysis and in vitro culture of Borrelia crocidurae. J Clin Microbiol 1999; 37: 2027–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordstrand A, Bunikis I, Larsson C, et al. Tickborne relapsing fever diagnosis obscured by malaria, Togo. Emerg Infect Dis 2007; 13: 117–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mooser H. The preservation of an egyptian strain of Borrelia crocidurae in Ornithodoros moubata. Acta Trop 1963; 20: 369–72 [PubMed] [Google Scholar]
- 13.Dirk Van Peenen PF. Arvicanthis niloticus Desmarest, 1822, a new host for Borrelia crocidurae in Egypt. East Afr Med J 1963; 40: 83–6 [PubMed] [Google Scholar]
- 14.Khalil GM, Helmy N, Hoogstraal H, et al. Seasonal dynamics of Ornithodoros (Pavlovskyella) erraticus (Acari: Ixodoidea: Argasidae) and the spirochete Borrelia crocidurae in Egypt. J Med Entomol 1984; 21: 536–9 [DOI] [PubMed] [Google Scholar]
- 15.Chatel G, Gulletta M, Matteelli A, et al. Short report: Diagnosis of tick-borne relapsing fever by the quantitative buffy coat fluorescence method. Am J Trop Med Hyg 1999; 60: 738–9 [DOI] [PubMed] [Google Scholar]
- 16.Shamaei-Tousi A, Martin P, Bergh A, et al. Erythrocyte-aggregating relapsing fever spirochete Borrelia crocidurae induces formation of microemboli. J Infect Dis 1999; 180: 1929–38 [DOI] [PubMed] [Google Scholar]
- 17.Cadavid D, Barbour AG. Neuroborreliosis during relapsing fever: review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clin Infect Dis 1998; 26: 151–64 [DOI] [PubMed] [Google Scholar]
- 18.Schwan TG, Schrumpf ME, Hinnebusch BJ, et al. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol 1996; 34: 2483–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukunaga M, Ushijima Y, Aoki LY, et al. Detection of Borrelia duttonii, a tick-borne relapsing fever agent in central Tanzania, within ticks by flagellin gene-based nested polymerase chain reaction. Vector Borne Zoonotic Dis 2001; 1: 331–8 [DOI] [PubMed] [Google Scholar]
- 20.Halperin T, Orr N, Cohen R, et al. Detection of relapsing fever in human blood samples from Israel using PCR targeting the glycerophosphodiester phosphodiesterase (GlpQ) gene. Acta Trop 2006; 98: 189–95 [DOI] [PubMed] [Google Scholar]
- 21.Scott JC. Typing African relapsing fever spirochetes. Emerg Infect Dis 2005; 11: 1722–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasin T, Davidovitch N, Cohen R, et al. Postexposure treatment with doxycycline for the prevention of tick-borne relapsing fever. N Engl J Med 2006; 355: 148–55 [DOI] [PubMed] [Google Scholar]