Abstract
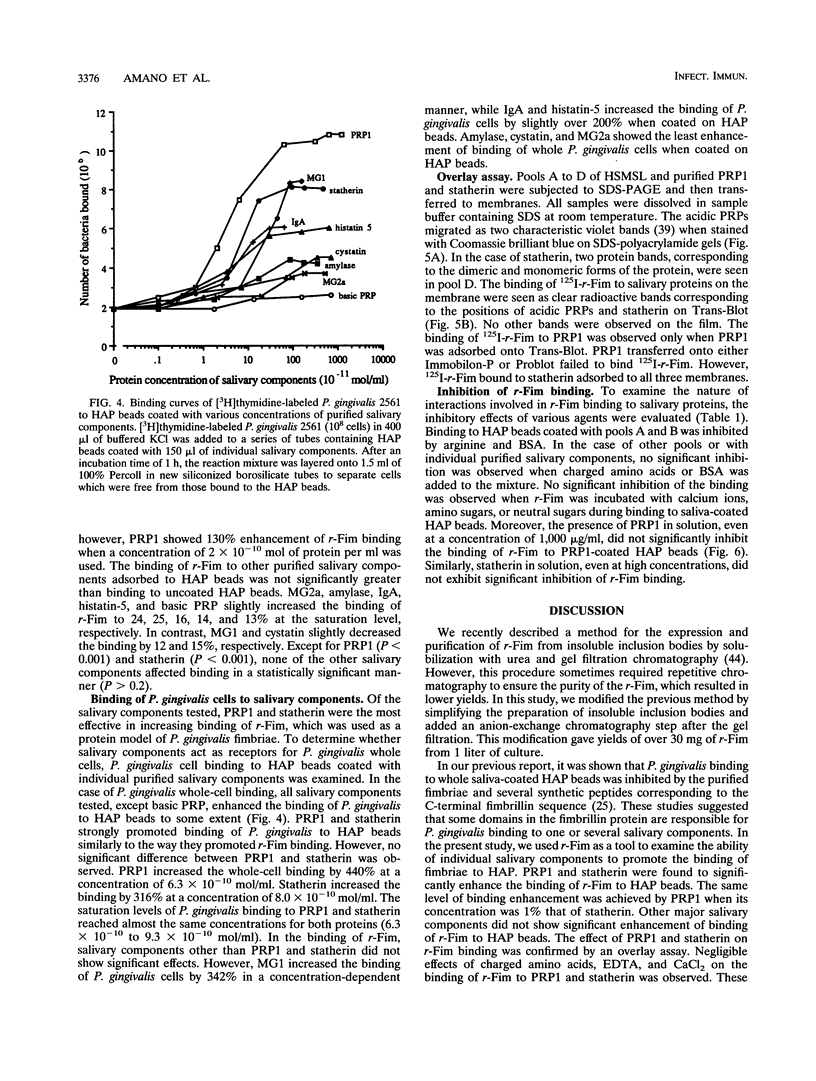
Fimbriae are considered important in the adherence and colonization of Porphyromonas gingivalis in the oral cavity. It has been demonstrated that purified fimbriae bind to whole human saliva adsorbed to hydroxyapatite (HAP) beads, and the binding appears to be mediated by specific protein-protein interactions. Recently, we expressed the recombinant fimbrillin protein (r-Fim) of P. gingivalis corresponding to amino acid residues 10 to 337 of the native fimbrillin (A. Sharma, H.T. Sojar, J.-Y. Lee, and R.J. Genco, Infect. Immun. 61:3570-3573, 1993). We examined the ability of individual salivary components to promote the direct attachment of r-Fim to HAP beads. Purified r-Fim was radiolabeled with 125I and incubated with HAP beads which were coated with saliva or purified individual salivary components. Whole, parotid, and submandibular-sublingual salivas increased the binding of 125I-r-Fim to HAP beads. Submandibular-sublingual saliva was most effective in increasing the binding of 125I-r-Fim to HAP beads (1.8 times greater than that to uncoated HAP beads). The binding of 125I-r-Fim to HAP beads coated with acidic proline-rich protein 1 (PRP1) or statherin was four and two times greater, respectively, than that to uncoated HAP beads. PRP1 and statherin molecules were also found to bind 125I-r-Fim in an overlay assay. The binding of intact P. gingivalis cells to HAP beads coated with PRP1 or statherin was also enhanced, by 5.4 and 4.3 times, respectively, over that to uncoated HAP beads. The interactions of PRP1 and statherin with 125I-r-Fim were not inhibited by the addition of carbohydrates or amino acids. PRP1 and statherin in solution did not show inhibitory activity on 125I-r-Fim binding to HAP beads coated with PRP1 or statherin. These results suggest that P. gingivalis fimbriae bind strongly through protein-protein interactions to acidic proline-rich protein and statherin molecules which coat surfaces.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennick A. Salivary proline-rich proteins. Mol Cell Biochem. 1982 Jun 11;45(2):83–99. doi: 10.1007/BF00223503. [DOI] [PubMed] [Google Scholar]
- Boyd J., McBride B. C. Fractionation of hemagglutinating and bacterial binding adhesins of Bacteroides gingivalis. Infect Immun. 1984 Aug;45(2):403–409. doi: 10.1128/iai.45.2.403-409.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Childs W. C., 3rd, Gibbons R. J. Selective modulation of bacterial attachment to oral epithelial cells by enzyme activities associated with poor oral hygiene. J Periodontal Res. 1990 May;25(3):172–178. doi: 10.1111/j.1600-0765.1990.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Beem J. E., Nesbitt W. E., Cisar J. O., Tseng C. C., Levine M. J. Pellicle receptors for Actinomyces viscosus type 1 fimbriae in vitro. Infect Immun. 1989 Oct;57(10):3003–3008. doi: 10.1128/iai.57.10.3003-3008.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. P., Kubiniec M. A., Yoshimura F., Genco R. J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988 Apr;170(4):1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. T., Klausen B., Sojar H. T., Bedi G. S., Sfintescu C., Ramamurthy N. S., Golub L. M., Genco R. J. Immunization with Porphyromonas (Bacteroides) gingivalis fimbriae protects against periodontal destruction. Infect Immun. 1992 Jul;60(7):2926–2935. doi: 10.1128/iai.60.7.2926-2935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Adherent interactions which may affect microbial ecology in the mouth. J Dent Res. 1984 Mar;63(3):378–385. doi: 10.1177/00220345840630030401. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Comparative hydrophobicities of oral bacteria and their adherence to salivary pellicles. Infect Immun. 1983 Sep;41(3):1190–1196. doi: 10.1128/iai.41.3.1190-1196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Adsorbed salivary acidic proline-rich proteins contribute to the adhesion of Streptococcus mutans JBP to apatitic surfaces. J Dent Res. 1989 Sep;68(9):1303–1307. doi: 10.1177/00220345890680090201. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988 Feb;56(2):439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I., Schlesinger D. H. Delineation of a segment of adsorbed salivary acidic proline-rich proteins which promotes adhesion of Streptococcus gordonii to apatitic surfaces. Infect Immun. 1991 Sep;59(9):2948–2954. doi: 10.1128/iai.59.9.2948-2954.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulbourne P. A., Ellen R. P. Evidence that Porphyromonas (Bacteroides) gingivalis fimbriae function in adhesion to Actinomyces viscosus. J Bacteriol. 1991 Sep;173(17):5266–5274. doi: 10.1128/jb.173.17.5266-5274.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hanazawa S., Hirose K., Ohmori Y., Amano S., Kitano S. Bacteroides gingivalis fimbriae stimulate production of thymocyte-activating factor by human gingival fibroblasts. Infect Immun. 1988 Jan;56(1):272–274. doi: 10.1128/iai.56.1.272-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Murakami Y., Hirose K., Amano S., Ohmori Y., Higuchi H., Kitano S. Bacteroides (Porphyromonas) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect Immun. 1991 Jun;59(6):1972–1977. doi: 10.1128/iai.59.6.1972-1977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I., Bennick A., Schlesinger D. H., Minaguchi K., Madapallimattam G., Schluckebier S. K. The primary structures of six human salivary acidic proline-rich proteins (PRP-1, PRP-2, PRP-3, PRP-4, PIF-s and PIF-f). Biochem J. 1988 Oct 1;255(1):15–21. doi: 10.1042/bj2550015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai H., Isogai E., Yoshimura F., Suzuki T., Kagota W., Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988;33(7):479–485. doi: 10.1016/0003-9969(88)90028-3. [DOI] [PubMed] [Google Scholar]
- Johnsson M., Levine M. J., Nancollas G. H. Hydroxyapatite binding domains in salivary proteins. Crit Rev Oral Biol Med. 1993;4(3-4):371–378. doi: 10.1177/10454411930040031601. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Sojar H. T., Bedi G. S., Genco R. J. Synthetic peptides analogous to the fimbrillin sequence inhibit adherence of Porphyromonas gingivalis. Infect Immun. 1992 Apr;60(4):1662–1670. doi: 10.1128/iai.60.4.1662-1670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ellen R. P., Hoover C. I., Felton J. R. Association of proteases of Porphyromonas (Bacteroides) gingivalis with its adhesion to Actinomyces viscosus. J Dent Res. 1991 Feb;70(2):82–86. doi: 10.1177/00220345910700021501. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E. C., Varughese K., Hay D. I. Effect of human salivary proteins on the precipitation kinetics of calcium phosphate. Calcif Tissue Int. 1979 Aug 24;28(1):7–16. doi: 10.1007/BF02441212. [DOI] [PubMed] [Google Scholar]
- Naito Y., Gibbons R. J. Attachment of Bacteroides gingivalis to collagenous substrata. J Dent Res. 1988 Aug;67(8):1075–1080. doi: 10.1177/00220345880670080301. [DOI] [PubMed] [Google Scholar]
- Naito Y., Tohda H., Okuda K., Takazoe I. Adherence and hydrophobicity of invasive and noninvasive strains of Porphyromonas gingivalis. Oral Microbiol Immunol. 1993 Aug;8(4):195–202. doi: 10.1111/j.1399-302x.1993.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Nishikata M., Yoshimura F., Nodasaka Y. Possibility of Bacteroides gingivalis hemagglutinin possessing protease activity revealed by inhibition studies. Microbiol Immunol. 1989;33(1):75–80. doi: 10.1111/j.1348-0421.1989.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Okuda K., Yamamoto A., Naito Y., Takazoe I., Slots J., Genco R. J. Purification and properties of hemagglutinin from culture supernatant of Bacteroides gingivalis. Infect Immun. 1986 Dec;54(3):659–665. doi: 10.1128/iai.54.3.659-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyston P. C., Handley P. S. Surface structures, haemagglutination and cell surface hydrophobicity of Bacteroides fragilis strains. J Gen Microbiol. 1990 May;136(5):941–948. doi: 10.1099/00221287-136-5-941. [DOI] [PubMed] [Google Scholar]
- Peros W. J., Etherden I., Gibbons R. J., Skobe Z. Alteration of fimbriation and cell hydrophobicity by sublethal concentrations of tetracycline. J Periodontal Res. 1985 Jan;20(1):24–30. doi: 10.1111/j.1600-0765.1985.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Raj P. A., Edgerton M., Levine M. J. Salivary histatin 5: dependence of sequence, chain length, and helical conformation for candidacidal activity. J Biol Chem. 1990 Mar 5;265(7):3898–3905. [PubMed] [Google Scholar]
- Ramasubbu N., Reddy M. S., Bergey E. J., Haraszthy G. G., Soni S. D., Levine M. J. Large-scale purification and characterization of the major phosphoproteins and mucins of human submandibular-sublingual saliva. Biochem J. 1991 Dec 1;280(Pt 2):341–352. doi: 10.1042/bj2800341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Buivids I. A., Ellen R. P. Adhesion of Actinomyces viscosus to Porphyromonas (Bacteroides) gingivalis-coated hexadecane droplets. J Bacteriol. 1991 Apr;173(8):2581–2589. doi: 10.1128/jb.173.8.2581-2589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco F. A., Bergey E. J., Reddy M. S., Levine M. J. Characterization of salivary alpha-amylase binding to Streptococcus sanguis. Infect Immun. 1989 Sep;57(9):2853–2863. doi: 10.1128/iai.57.9.2853-2863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Ellen R. P., Grove D. A. Bacteroides gingivalis-Actinomyces viscosus cohesive interactions as measured by a quantitative binding assay. Infect Immun. 1987 Oct;55(10):2391–2397. doi: 10.1128/iai.55.10.2391-2397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Sojar H. T., Lee J. Y., Genco R. J. Expression of a functional Porphyromonas gingivalis fimbrillin polypeptide in Escherichia coli: purification, physicochemical and immunochemical characterization, and binding characteristics. Infect Immun. 1993 Aug;61(8):3570–3573. doi: 10.1128/iai.61.8.3570-3573.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojar H. T., Lee J. Y., Bedi G. S., Cho M. I., Genco R. J. Purification, characterization and immunolocalization of fimbrial protein from Porphyromonas (bacteroides) gingivalis. Biochem Biophys Res Commun. 1991 Mar 15;175(2):713–719. doi: 10.1016/0006-291x(91)91624-l. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Haraszthy G. G., Zhang X. L., Levine M. J. Inhibition of Porphyromonas gingivalis adhesion to Streptococcus gordonii by human submandibular-sublingual saliva. Infect Immun. 1992 Jul;60(7):2598–2604. doi: 10.1128/iai.60.7.2598-2604.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Yamaji Y., Umemoto T. Correlation between cell-adherent activity and surface structure in Porphyromonas gingivalis. Oral Microbiol Immunol. 1992 Dec;7(6):357–363. doi: 10.1111/j.1399-302x.1992.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]